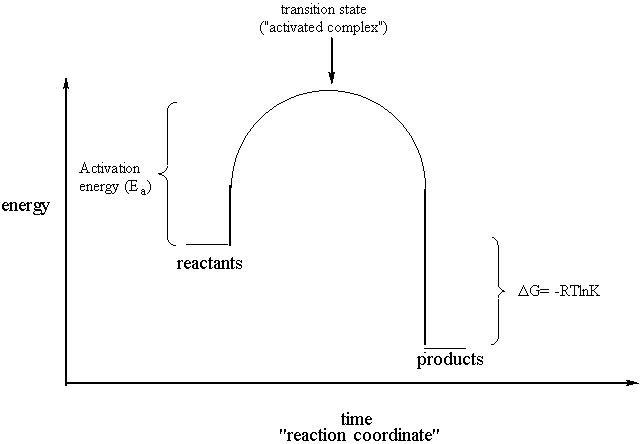
The Diagram Represents A Spontaneous Reaction Use The Diagram To Answer The Questions Below
The heat is on the product side of the equation which means that the heat leaves the reaction like the products. Endothermic exothermic what is the activation energy of the.
 Activation Energy And The Activated Complex Energy And
Activation Energy And The Activated Complex Energy And
Answer c is true because of the equation.
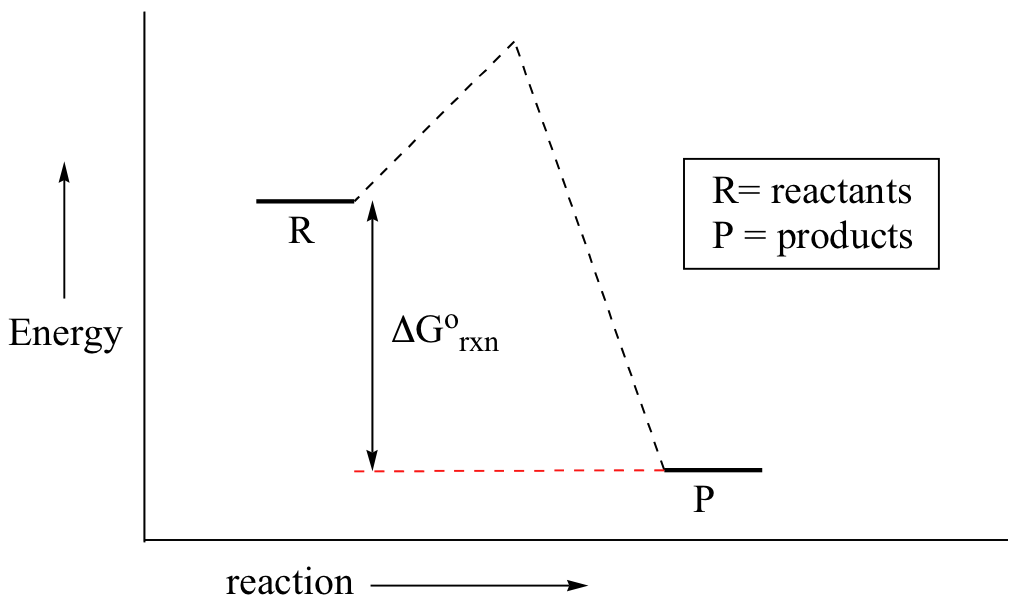
The diagram represents a spontaneous reaction use the diagram to answer the questions below. Answer b is also true because gibbs is measured as the difference between these two on a reaction coordinate diagram. Use the diagram that shows a fission nuclear reactor to answer the following question. The diagram represents a spontaneous reaction.
Is the reaction endothermic or exothermic. Let the red spheres represent element a the green ones element b and the blue ones element c. The diagram below represents a nuclear reaction in which a neutron bombards a heavy nucleus.
1which ionic equation represents a spontaneous reaction that can occur in a voltaic cell. The diagram below represents a spontaneous reaction deltag degree 0. If it was endothermic the heat would be absorbed to do the the reaction.
Start studying chemistry final exam review part 3 2014 2015. The diagram represents a spontaneous reaction. The following diagram represents an imaginary two step mechanism.
8base your answer to the following question on the. Endothermic exothermic what is the activation energy of the reaction. Use the diagram to answer the questions below.
14the diagram below represents an electrochemical cell. Drag the labels to the correct bins. Use the diagram to answer the questions below.
Aat the anode bat the cathode cin the salt bridge din the wire. Use the diagram to answer the questions below. Chemical equation question with a diagram.
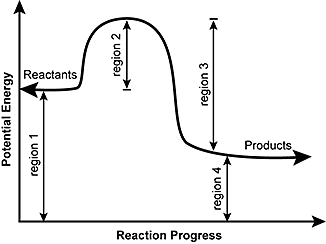
Sometimes a teacher finds it necessary to ask questions about pe diagrams that involve actual potential energy values. Solved the diagram shown shows the reaction profile. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction.
Learn vocabulary terms and more with flashcards games and other study tools. This first video takes you through all the basic parts of the pe diagram. The natural log of a number less than one is a negative number so if k is less than one gibbs will turn positive.
Use the diagram to answer the questions below. The diagram represents a spontaneous reaction. Posted one year ago.
Uncatalyzed rxn delta g degree standard free e of activation products reactants catalyzed rxn. The diagram represents a spontaneous reaction. Is the reaction endothermic or exothermic.
 Solved The Diagram Represents A Spontaneous Reaction Use
Solved The Diagram Represents A Spontaneous Reaction Use
 Answer The Diagram Represents A Spontaneo Clutch Prep
Answer The Diagram Represents A Spontaneo Clutch Prep
Osa Energy Weighted X Ray Dark Field Imaging
 Potential Energy Diagrams Chemistry Catalyst Endothermic Exothermic Reactions
Potential Energy Diagrams Chemistry Catalyst Endothermic Exothermic Reactions
 Should Job Applicants Be Excited Or Calm The Role Of
Should Job Applicants Be Excited Or Calm The Role Of
Ch103 Chapter 7 Chemical Reactions In Biological Systems
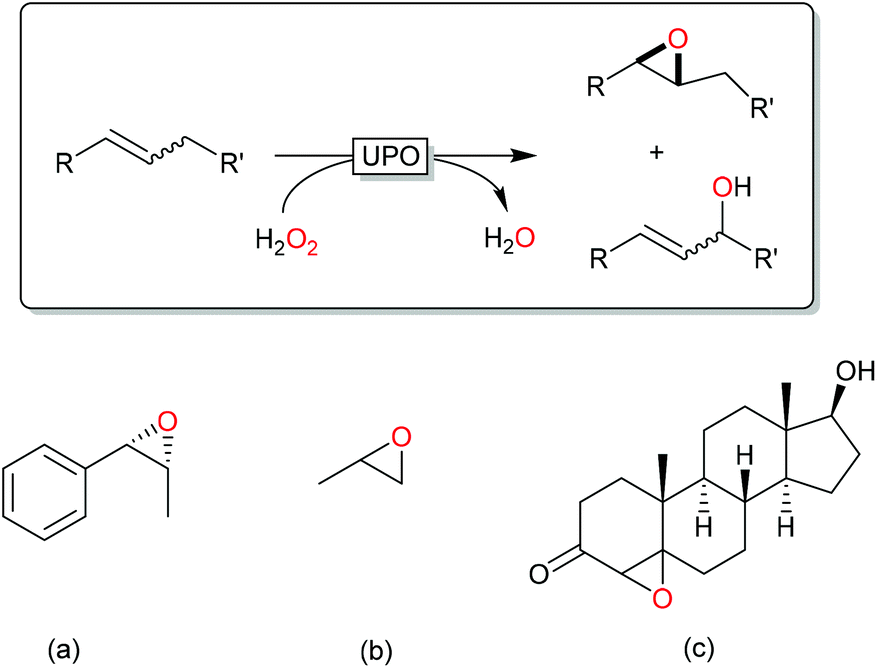 Hydrogen Peroxide Driven Biocatalysis Green Chemistry Rsc
Hydrogen Peroxide Driven Biocatalysis Green Chemistry Rsc
 Activation Energy And The Activated Complex Energy And
Activation Energy And The Activated Complex Energy And
 Activation Energy And Temperature Dependence Boundless
Activation Energy And Temperature Dependence Boundless
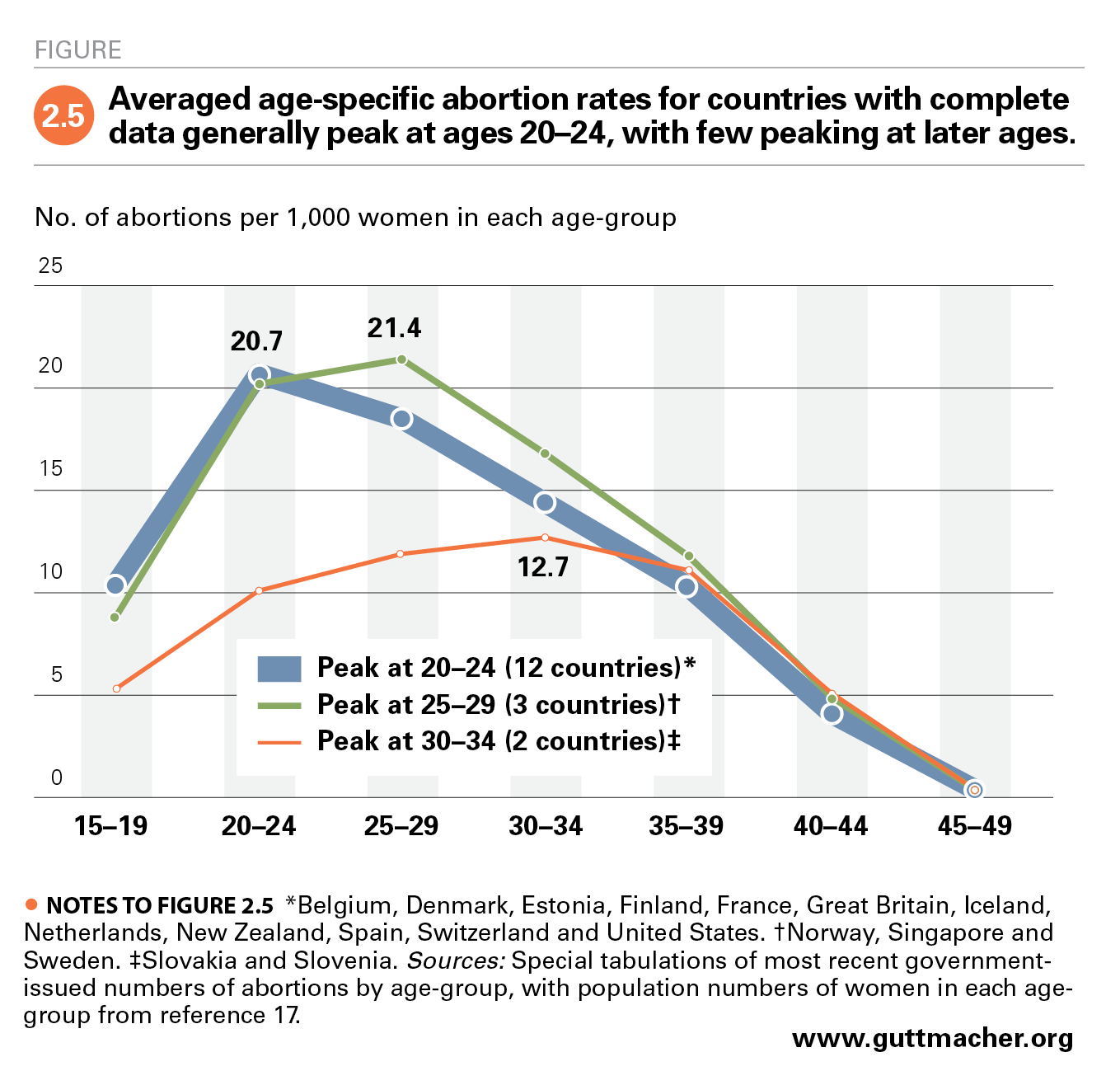 Abortion Worldwide 2017 Uneven Progress And Unequal Access
Abortion Worldwide 2017 Uneven Progress And Unequal Access
 Artificial Love Design For Emotional Intelligence
Artificial Love Design For Emotional Intelligence
 7 6 Energetics And Kinetics Chemistry Libretexts
7 6 Energetics And Kinetics Chemistry Libretexts
 Spontaneous Generation Of Hydrogen Peroxide From Aqueous
Spontaneous Generation Of Hydrogen Peroxide From Aqueous
 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
 Solved The Diagram Represents A Spontaneous Reaction Use
Solved The Diagram Represents A Spontaneous Reaction Use
 Free Energy Landscape And Molecular Pathways Of Gas Hydrate
Free Energy Landscape And Molecular Pathways Of Gas Hydrate
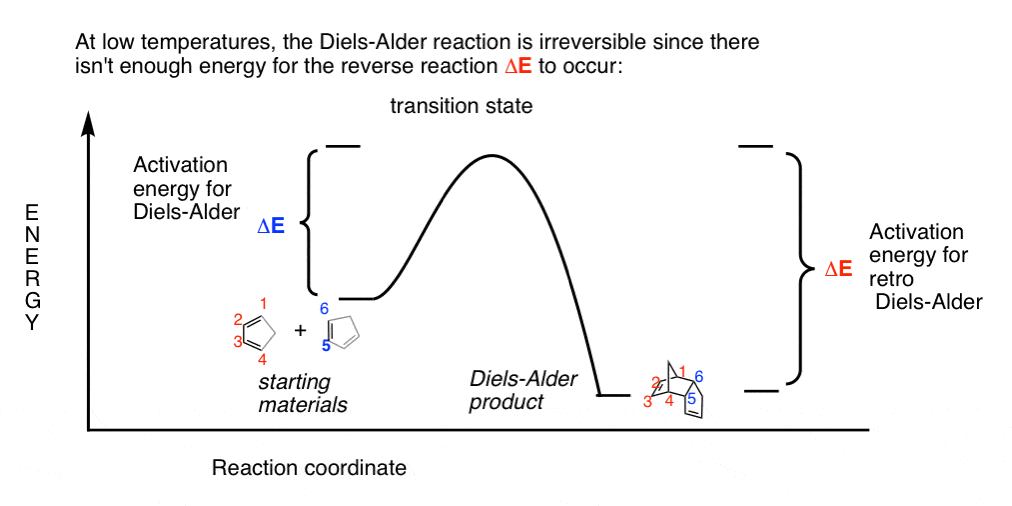 Kinetic And Thermodynamic Control In The Diels Alder Reaction
Kinetic And Thermodynamic Control In The Diels Alder Reaction
 The Diagram Represents A Spontaneous Reaction Use The Diagram To Answer The Questions Below A Is The Reaction Endothermic Or Exothermic B What Is The Activation Energy Of The Reaction
The Diagram Represents A Spontaneous Reaction Use The Diagram To Answer The Questions Below A Is The Reaction Endothermic Or Exothermic B What Is The Activation Energy Of The Reaction


Belum ada Komentar untuk "The Diagram Represents A Spontaneous Reaction Use The Diagram To Answer The Questions Below"
Posting Komentar