Energy Diagram Catalyzed Vs Uncatalyzed Reaction
Substrate binding by serine proteases. Uncatalyzed reaction activation energy substrate s catalyzed reaction product p.
The Mechanism Of Enzyme Catalysis
Models of enzyme substrate interaction.
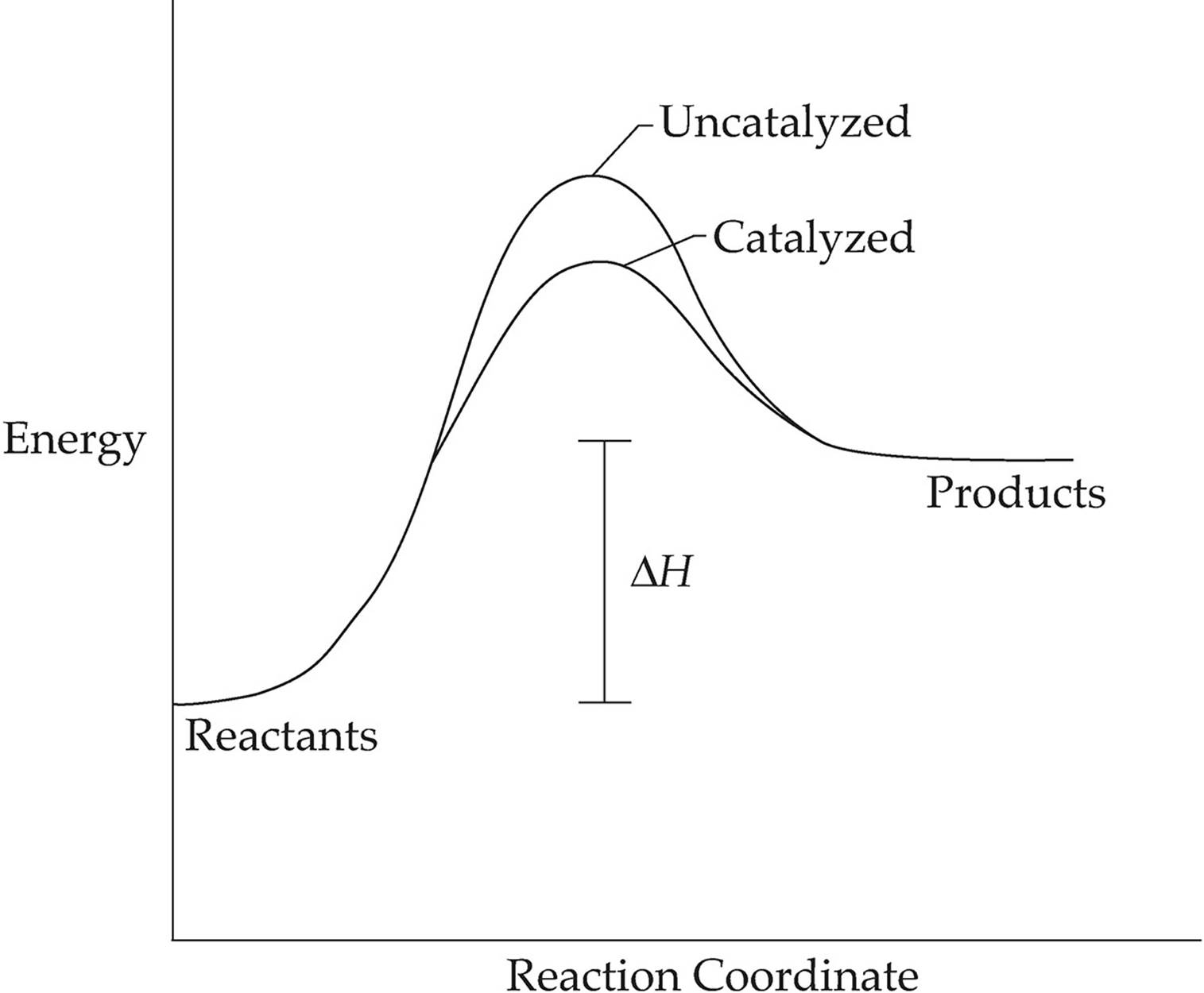
Energy diagram catalyzed vs uncatalyzed reaction. Enzymatic catalysis of a reaction between two substrates. Lots of great tidbits in this one. The energy diagram for a reaction model consisting of one enzyme one substrate and one product is depicted in many books where it is compared with that for the uncatalyzed reaction.
The δh for the reactions is the same. Draw the free energy diagram of catalyzed vs. Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction.
Energy diagrams for catalyzed and uncatalyzed reactions. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Because a catalyst decreases the height of the energy barrier its presence increases the reaction rates of both the forward and the reverse reactions by the same amount.
Name the five general catalytic mechanisms. This problem has been solved. Its time to learn a little more about a chemical reaction.
Type of reaction catalyzed. You will place all the labels in part a. Catalyzed vs uncatalyzed reaction mechanism by jessie a.
How do molecules have to be arranged and how much energy do they have to collide with. Potential energy diagram of catalyzed vs uncatalyzed reaction pathway. Uncatalyzed reaction has a higher activation energy because there is no enzyme present in the.
Learn vocabulary terms and more with flashcards games and other study tools. Gibbs free energy reaction coordinate profiles found in some textbooks. Key is licensed under a creative commons attribution noncommercial sharealike 40 international license except where otherwise noted.
Label the energy diagram and answer the question that follows. The only difference between a catalyzed reaction and an uncatalyzed reaction is that the activation energy is different. Catalyzed reaction has a lower activation energy because there is an enzyme present in the reaction.
There is no effect on the energy of the reactants or the products. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
1 acid base catalysis. Nevertheless because of its lower e a the reaction rate of a catalyzed reaction is faster than the reaction rate of the uncatalyzed reaction at the same temperature. Enzymes are important molecules in biochemistry that catalyze reactions.
 4 Energy Diagram Of Case 1 In The Oxidation Of Diols To A
4 Energy Diagram Of Case 1 In The Oxidation Of Diols To A
Section 13 3 The Rate Of A Reaction
 Please Refer To The Charts A What Is Th Clutch Prep
Please Refer To The Charts A What Is Th Clutch Prep
 An Energy Profile Diagram Of An Uncatalyzed Reaction Grey
An Energy Profile Diagram Of An Uncatalyzed Reaction Grey
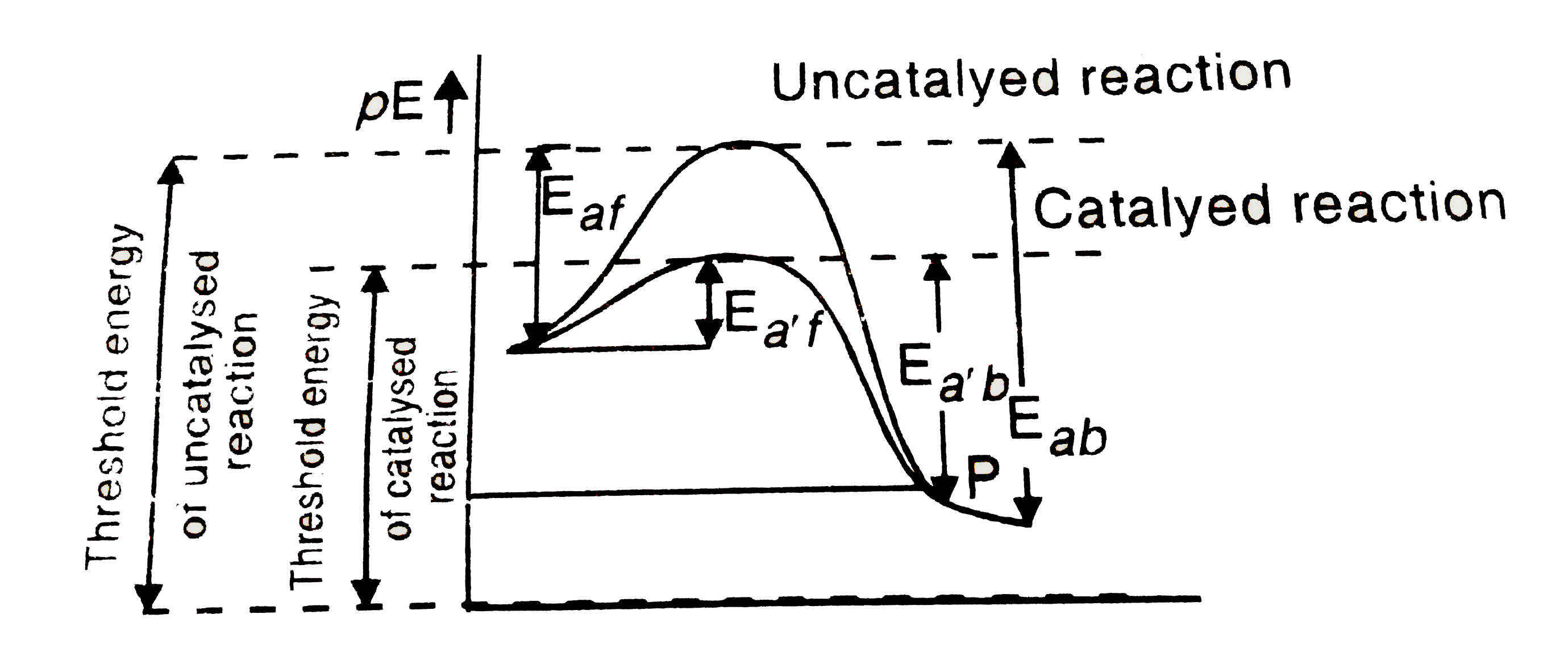 The Energy Profile Diagrams Are Very Important Tool Through
The Energy Profile Diagrams Are Very Important Tool Through
Bio Un2005 2401x 2017 Lec 7 L Chasin September 26 2017
Energy And Enzymes Biology 1511 Biological Principles
 Screen Shot 2017 04 13 At 3 38 13 Pm Png Enzymes Are
Screen Shot 2017 04 13 At 3 38 13 Pm Png Enzymes Are
 Figure 2 From Encyclopedia Of Life Support Systems Eolss
Figure 2 From Encyclopedia Of Life Support Systems Eolss
Enzymes Catalysis And Kinetics
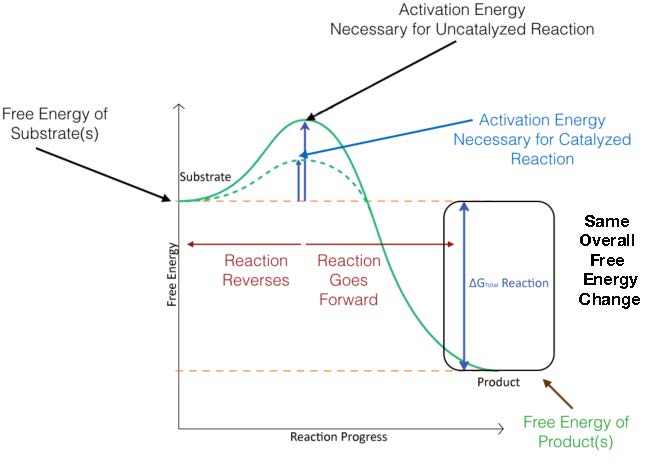 4 1 Basic Principles Of Catalysis Biology Libretexts
4 1 Basic Principles Of Catalysis Biology Libretexts
 Catalysts And Energy Diagrams Chemical Reactions Energy
Catalysts And Energy Diagrams Chemical Reactions Energy
 Mechanism Of Reaction And Catalysis Rate And Extent Of
Mechanism Of Reaction And Catalysis Rate And Extent Of

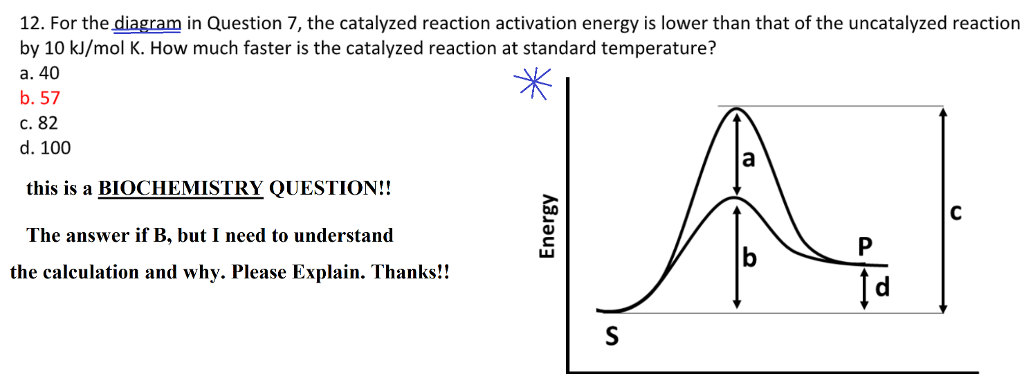 Solved 12 For The Diagram In Question 7 The Catalyzed R
Solved 12 For The Diagram In Question 7 The Catalyzed R
 Rates And Mechanisms Of Chemical Reactions Ppt Download
Rates And Mechanisms Of Chemical Reactions Ppt Download
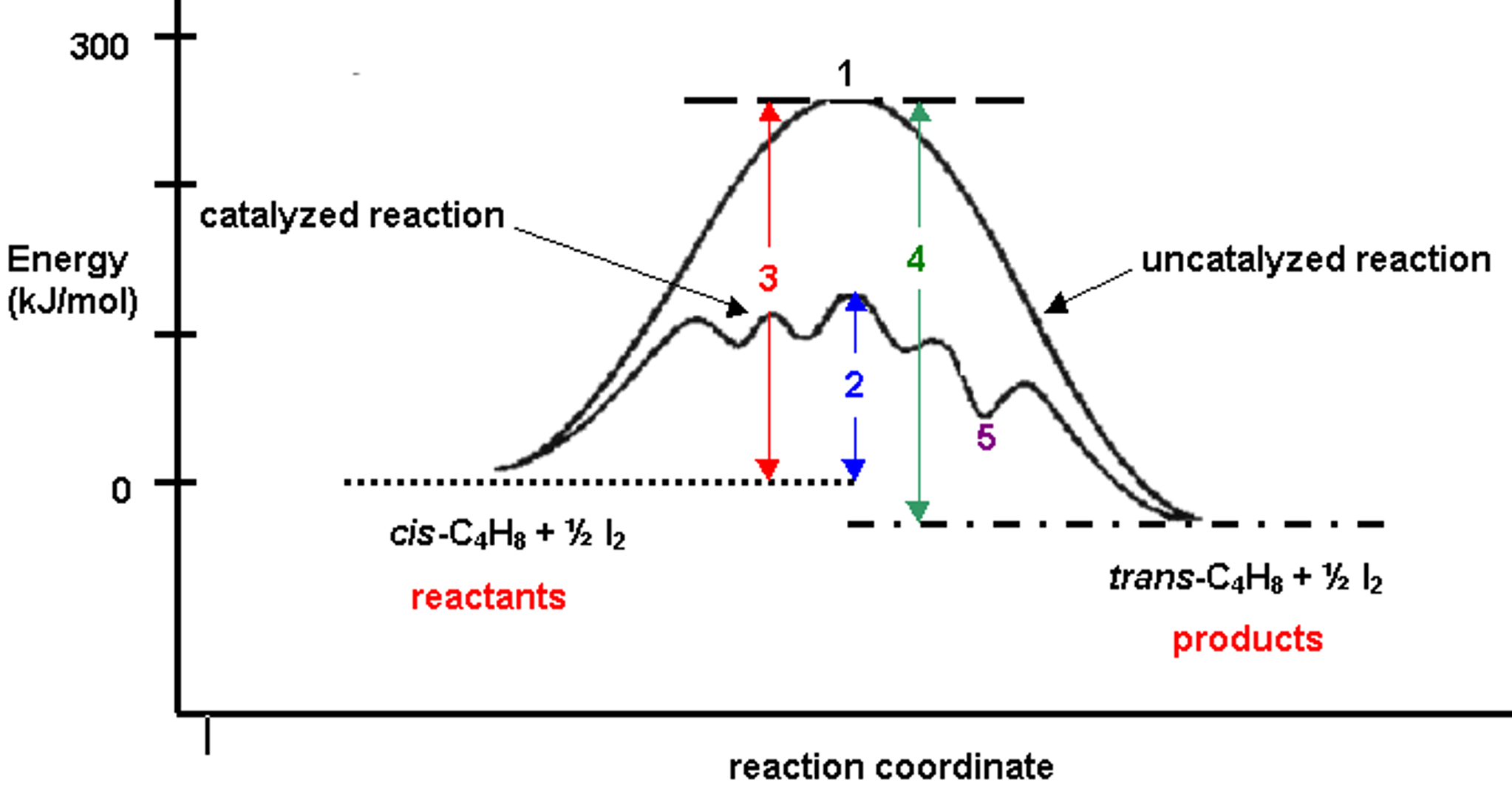 Solved The Diagram Shown Above Shows The Reaction Profile
Solved The Diagram Shown Above Shows The Reaction Profile
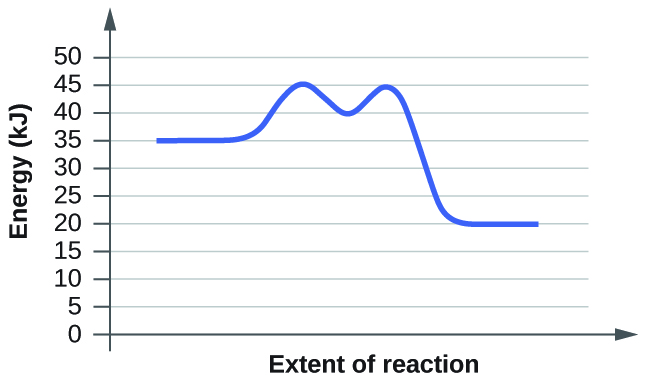


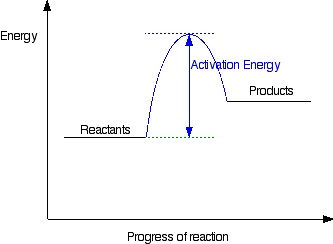

Belum ada Komentar untuk "Energy Diagram Catalyzed Vs Uncatalyzed Reaction"
Posting Komentar