The Diagram Below Represents A Spontaneous Reaction δg
This problem has been solved. Science chemistry video lessons exam reviews acs video solutions solutions library homework help.
 Spontaneous Mirror Symmetry Breaking And Origin Of
Spontaneous Mirror Symmetry Breaking And Origin Of
Drag the labels to the correct bins.
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)
The diagram below represents a spontaneous reaction δg. Ron rusay a reaction coordinate energy diagram thermodynamic quantities gibbs standard free energy change δgo enthalphy δho. Uncatalyzed rxn delta g degree standard free e of activation products reactants catalyzed rxn. The diagram below represents a spontaneous reaction δg institution.
Chem1101 2014 j 12 june 2014 the diagram below represents this reaction involves an increase in the number of moles of gas so δs will be positive as δh δg tδs this means that δh δg for the other reactions there is a decrease in the number of moles of has so δs is negative δh δg tδs this means that δh δg. Enzyme catalyzed reactions release more free energy than noncatalyzed reactions. The reaction always goes in the direction toward chemical equilibrium.
The diagram represents a spontaneous reaction. If you cant find your institution please check your spelling and do not use abbreviations. The free energy change of the reaction is opposite from the reaction that occurs in the absence of the enzyme.
The diagram below represents a spontaneous reaction deltag 0. Use the diagram to answer the questions below. The following diagram represents an imaginary two step mechanism.
Let the red spheres represent element a the green ones element b and the blue ones element c. Chemical equation question with a diagram. Watch the video solution for the question.
The figure below represents the spontaneous reaction of h2 shaded spheres with o2 unshaded spheres to. Drag the labels to the correct bins. What is the activation energy of the reaction.
The heat given off or absorbed during a reaction entropy δso. Drag the labels to the correct bins. The diagram below represents a spontaneous re.
Is the reaction endothermic or exothermic. Learn vocabulary terms and more with flashcards games and other study tools. Mgf2s mg2aq 2 f aq.
What is the relationship between δg and the δgf for the reaction below. Organic chemistry video lessons exam reviews acs video solutions solutions library. The diagram below represents a spontaneous reaction deltag degree 0.
Enzyme catalyzed reactions require energy to activate the enzyme. A measure of freedom of motion δgo δho tδso δgδhδs δe are state. Start studying i found you ms new booty.
Energy Enzymes And Catalysis Problem Set
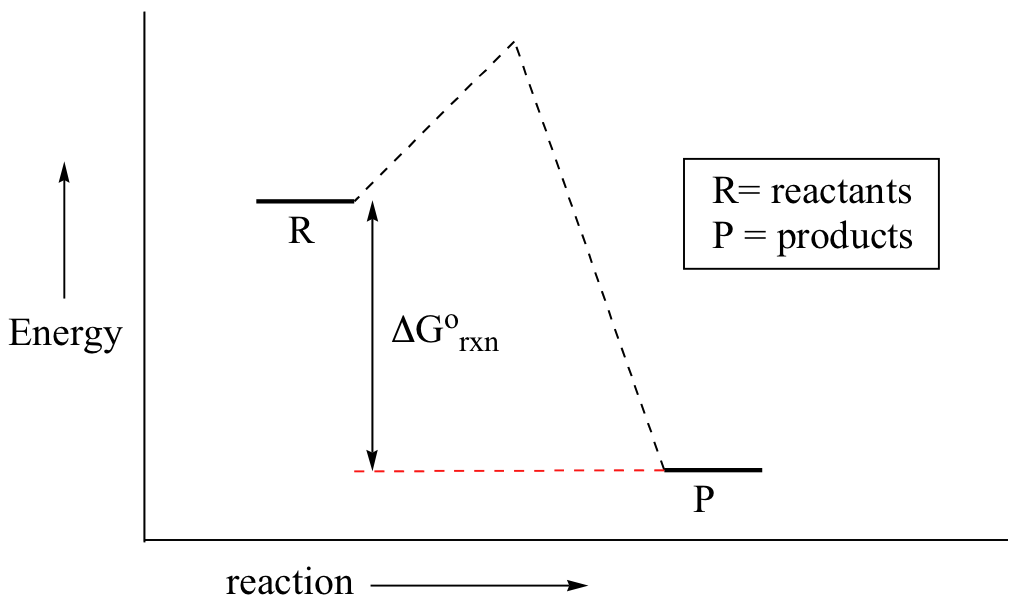 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png) Endergonic Vs Exergonic Reactions And Processes
Endergonic Vs Exergonic Reactions And Processes
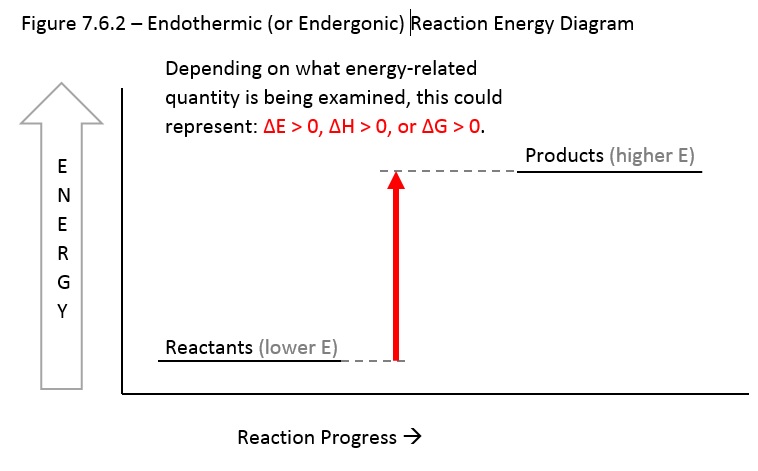 7 6 Energetics And Kinetics Chemistry Libretexts
7 6 Energetics And Kinetics Chemistry Libretexts
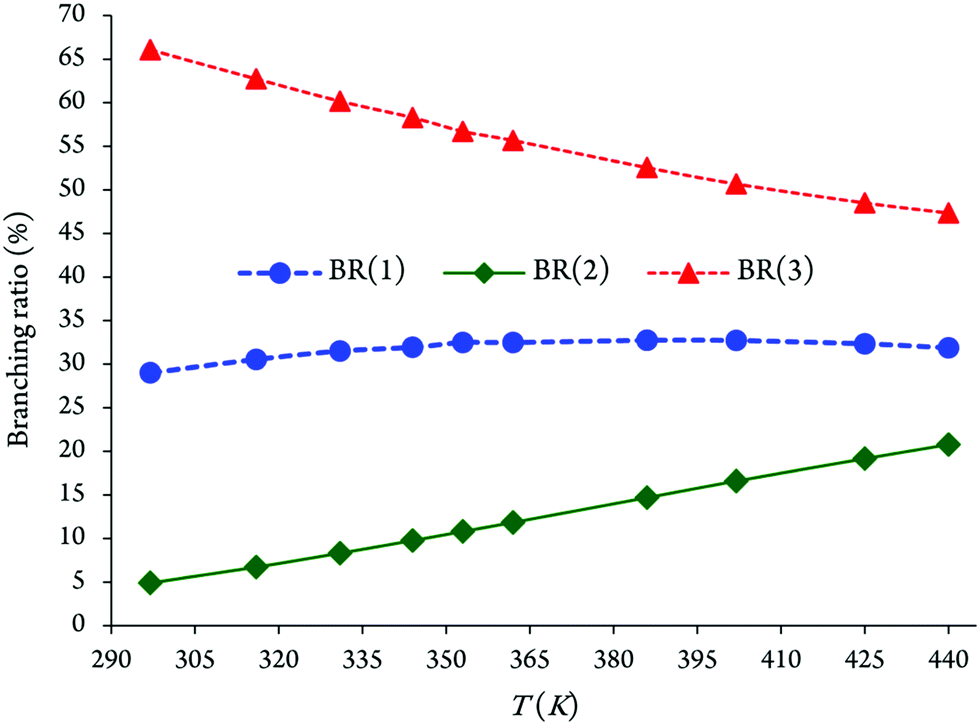 Atmospheric Oxidation Reactions Of Imidazole Initiated By
Atmospheric Oxidation Reactions Of Imidazole Initiated By
 Spontaneous Mirror Symmetry Breaking And Origin Of
Spontaneous Mirror Symmetry Breaking And Origin Of
Ch103 Chapter 7 Chemical Reactions In Biological Systems
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png) Endergonic Vs Exergonic Reactions And Processes
Endergonic Vs Exergonic Reactions And Processes

Ch103 Chapter 7 Chemical Reactions In Biological Systems
 Please Help Me With The Chemistry Problem Below Yahoo Answers
Please Help Me With The Chemistry Problem Below Yahoo Answers
 Spontaneous Reaction Definition Examples Video Lesson
Spontaneous Reaction Definition Examples Video Lesson
 7 6 Energetics And Kinetics Chemistry Libretexts
7 6 Energetics And Kinetics Chemistry Libretexts
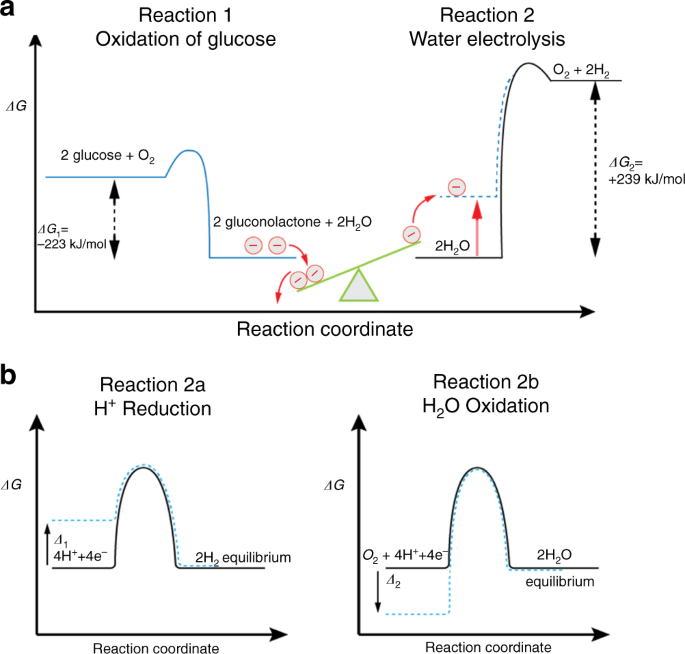 Uphill Production Of Dihydrogen By Enzymatic Oxidation Of
Uphill Production Of Dihydrogen By Enzymatic Oxidation Of
Solved The Figure Represents The Spontaneous Deposition O
Chem1101 2014 J 12 June 2014 The Diagram Below Represents
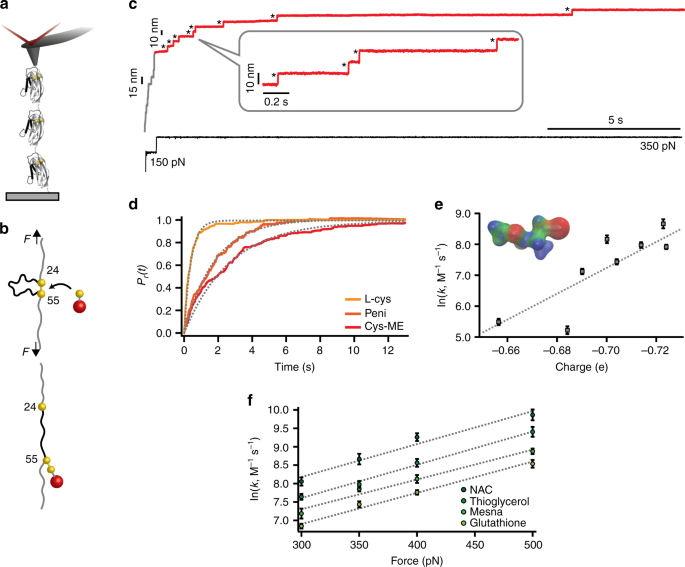 Forcing The Reversibility Of A Mechanochemical Reaction
Forcing The Reversibility Of A Mechanochemical Reaction
Simkinet A Free Educational Tool Based On An Electrical
 Quia Ap Chapter 8 An Introduction To Metabolism Detailed
Quia Ap Chapter 8 An Introduction To Metabolism Detailed
 Gibbs Free Energy Dg Analysis For The Naoh Sodium Oxygen
Gibbs Free Energy Dg Analysis For The Naoh Sodium Oxygen



Belum ada Komentar untuk "The Diagram Below Represents A Spontaneous Reaction δg"
Posting Komentar