Electron Dot Diagram For Ch4
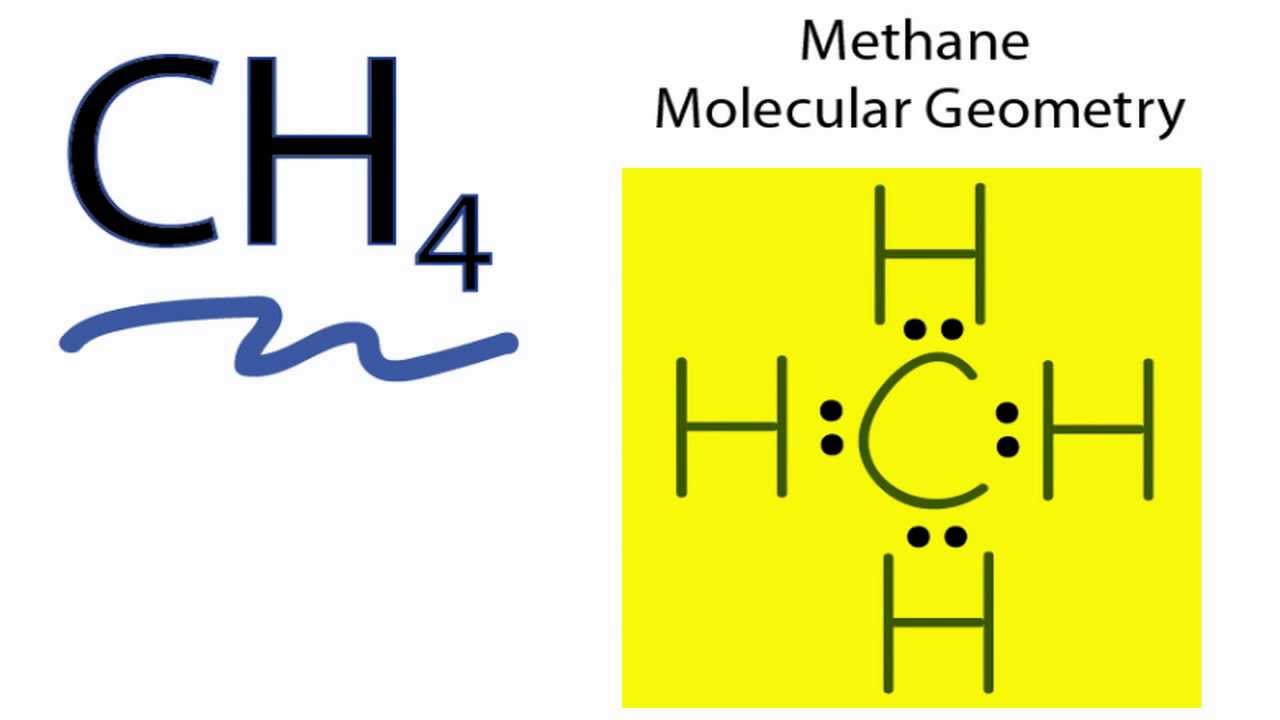
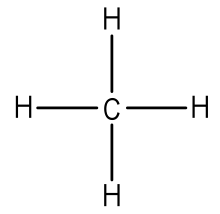
Each atom in the bond has a full valence with carbon having access to eight electrons and each hydrogen having access to two this is why hydrogen only needs twothe covalent bonds between the c and the h are similar to the ones formed between two hs. Ch4 or methanes molecular formula is given as ch4.
Topic Lewis Dot Diagrams For Ionic Compounds
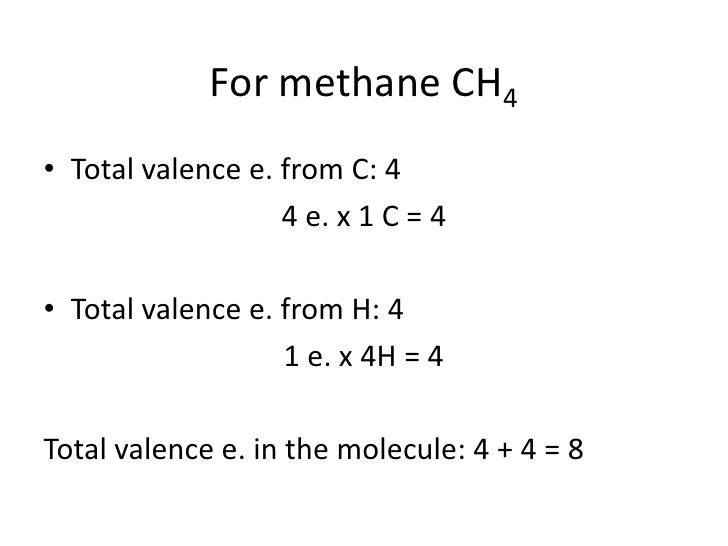
Methane for the ch4 lewis structure calculate the total number of valence electrons for the ch4 molecule ch4 has 8.

Electron dot diagram for ch4. The number of valence electrons. Some possible way to show the structure of ch4 are its electron dot diagram or structural formula. You could also represent the bonds as dots between the two atoms but this may be confused with the lone pair electrons on the nitrogen.
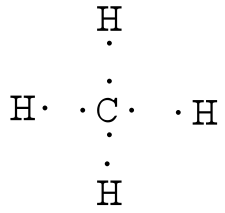
The lewis dot structure for ch 4 shows the number of valence electrons around each atom. This symbol represents the nucleus of the atom and each of the four sides. Note that hydrogen atoms always go on the outside of a lewis dot structure.
A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles. This is because they can share a maximum of two electrons. Each dot represents a valence electron.
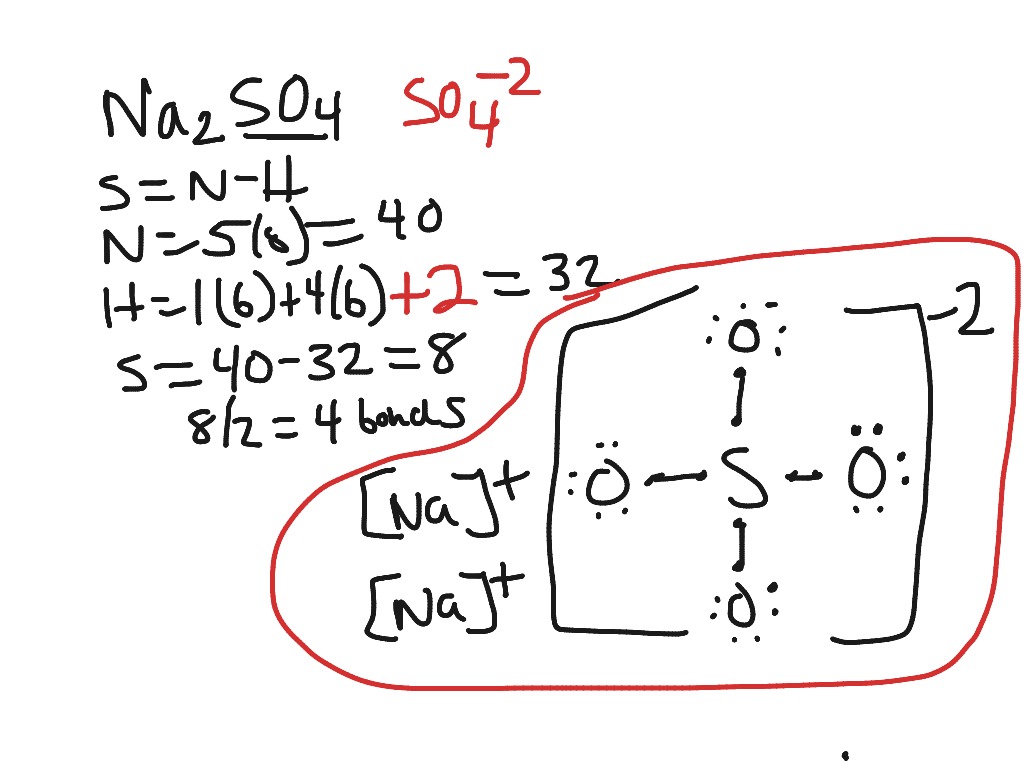
In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5. The lewis dot structure for ch4 is shown above. Explains how to draw the lewis dot structure for ch 4 methane.
Drawing the lewis structure for ch 4. The lewis dot structure for nh3 ammonia is shown above. The structural formula is a graphical.
Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper. These kinds of structures can also be shown by representing each of the bonds with two dots. The ch 4 lewis structure is one of the most frequently tested lewis structures.
Ch4 or methanes molecular formula is given as ch4. A step by step explanation of how to draw the ch4 lewis dot structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell.
This info can then be used to determine the lewis dot structure. For ch 4 you have a total of 8 total valence electrons. There is an easy way and a formal way to draw the lewis structure of ch 4.
Some possible way to show the structure of ch4 are its electron dot diagram or structural formula. Drawing the lewis structure for ch 4 named methane requires only single bondsits one of the easier lewis structures to draw. Looking at the ch4 lewis structure we can see that there are four hydrogen h atoms attached.
The structural formula is a graphical.
 Showme Lewis Electron Dot Diagram For Ch4
Showme Lewis Electron Dot Diagram For Ch4
 The Lewis Dot Structure For Ch4 Makethebrainhappy
The Lewis Dot Structure For Ch4 Makethebrainhappy
 How To Draw Methane Ch4 Lewis Structure
How To Draw Methane Ch4 Lewis Structure
What Is The Formation Of A Ch4 Molecule With The Lewis Dot
C Draw Electron Dot Structure Of I H2o Ii Ch4 Iii
 Ch4 Molecular Geometry Shape And Bond Angles
Ch4 Molecular Geometry Shape And Bond Angles
 The Lewis Dot Structure For Nh3 Makethebrainhappy
The Lewis Dot Structure For Nh3 Makethebrainhappy
 Multimedia Represent Bonding With Lewis Dot Diagrams
Multimedia Represent Bonding With Lewis Dot Diagrams
 Lewis Structure Custom Paper Example
Lewis Structure Custom Paper Example
Draw Electron Dot Structure Of Ch4 Science Carbon And
 Draw The Electron Dot Structure Of Ch4 Brainly In
Draw The Electron Dot Structure Of Ch4 Brainly In
 Solved In The Space Provided Below Draw Electron Dot Dia
Solved In The Space Provided Below Draw Electron Dot Dia
 The Lewis Dot Structure For Ch4 Makethebrainhappy
The Lewis Dot Structure For Ch4 Makethebrainhappy
 Lewis Dot Symbols And Lewis Structures Boundless Chemistry
Lewis Dot Symbols And Lewis Structures Boundless Chemistry
 Cl2 Lewis Structure How To Draw The Dot Structure For Cl2
Cl2 Lewis Structure How To Draw The Dot Structure For Cl2
 How Can The Electron Dot Structure For Ch4 Be Determined
How Can The Electron Dot Structure For Ch4 Be Determined
Covalent Bonding Electron Dot Structures Worksheet Answers
 Solved Data Sheet Molecular Geometry Lewis Structure Elec
Solved Data Sheet Molecular Geometry Lewis Structure Elec
 Draw The Electron Dot Structure Of Co2 Ch4 S8 Brainly In
Draw The Electron Dot Structure Of Co2 Ch4 S8 Brainly In
 Lewis Structures Single Double Triple Bonds Video
Lewis Structures Single Double Triple Bonds Video
 How To Draw Methane Ch4 Lewis Structure
How To Draw Methane Ch4 Lewis Structure
 How To Determine The Lewis Structure For Ch4 Quora
How To Determine The Lewis Structure For Ch4 Quora


Belum ada Komentar untuk "Electron Dot Diagram For Ch4"
Posting Komentar