Normal Boiling Point On Phase Diagram
If u watch american idol give me a star. It is therefore called the triple point of the substance and it represents the only point in the phase diagram in which all three states are in equilibrium.
A normal melting point at 20c.

Normal boiling point on phase diagram. A normal boiling point at 150c. How do you estimate the normal boiling point of the substance using a phase diagram. Learn the phase changes of matter.
How do you estimate the normal boiling point of the substance using a phase diagram. A ab b ac. In the generic phase diagram shown below you can see three curves that separate phases colour coded space on the diagram.
Do u think simon is good fo the show. The solid liquid line is normal meaning positive sloping. Estimate the normal freezing point of the substance.
Tell me who u want to win. And a critical point at 5 atm and 1000c. A phase diagram is a lot of points at differing temperatures and pressures.
Imagine a substance with the following points on the phase diagram. On the phase diagram shown to the right segment corresponds to the conditions of temperature and pressure under which the solid and the gas of the substance are in equilibrium. Know the density of air at stp.
For this complete the following. Point b in this phase diagram represents the only combination of temperature and pressure at which a pure substance can exist simultaneously as a solid a liquid and a gas. The boiling point in chemistry is affected by atmospheric pressure.
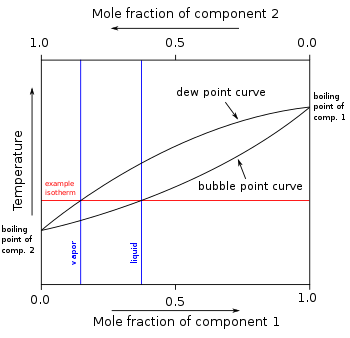
The normal boiling point is just one point where the vapourliquid equilibrium line occurs at one atmosphere pressure. It represents the equilibrium between the liquid and gas phases. Estimate the normal boiling point of the substance.
This is the definition of the normal melting point as the term is used in science and engineering. Figure 1124 general shape for a phase diagram of a system exhibiting three phases. The point on this curve where the vapor pressure is 1 atm is the normal boiling point of the substance.
A triple point at 5 atm and 5c. Gas liquid and solid. The line from a to b is the vapor pressure curve of the liquid.
What is the pressure of the substance at the triple. For a certain substance the normal melting point is 60oc the normal boiling point is.
 Answer The Following Questions Based On Th Clutch Prep
Answer The Following Questions Based On Th Clutch Prep
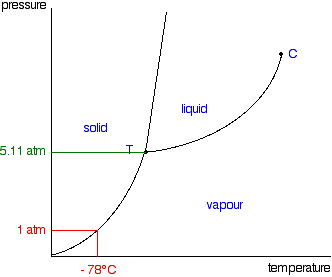 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
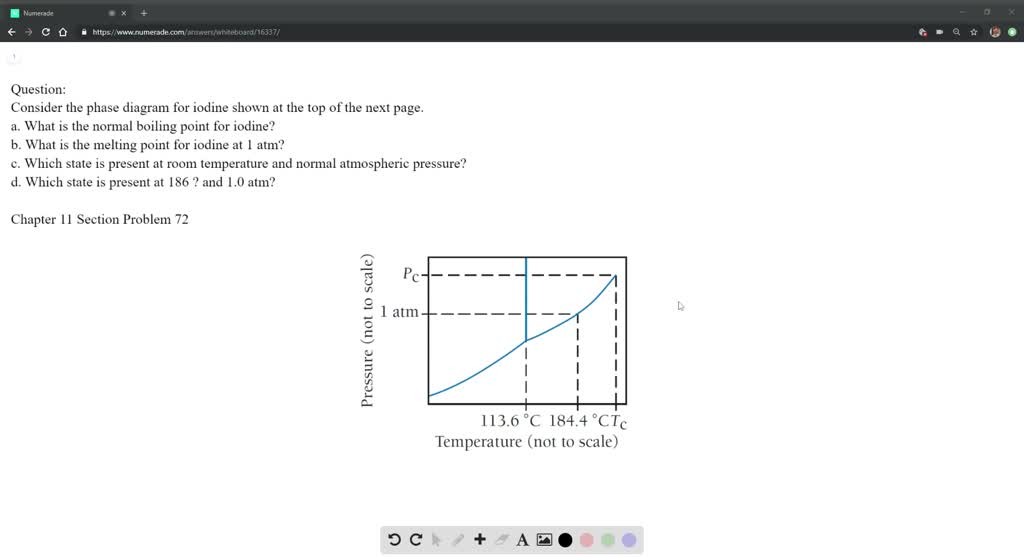 Solution For Consider The Phase Diagram For Iodine Shown At
Solution For Consider The Phase Diagram For Iodine Shown At
 Vapor Pressure 1atm 760 Mmhg 101 3kpa B Is A Gas B Is
Vapor Pressure 1atm 760 Mmhg 101 3kpa B Is A Gas B Is
Phase Diagram Worksheet 2 Name Period
10 13 Phase Diagrams Chemistry Libretexts
 A What Is The Normal Boiling Point For Iodine B What Is The
A What Is The Normal Boiling Point For Iodine B What Is The
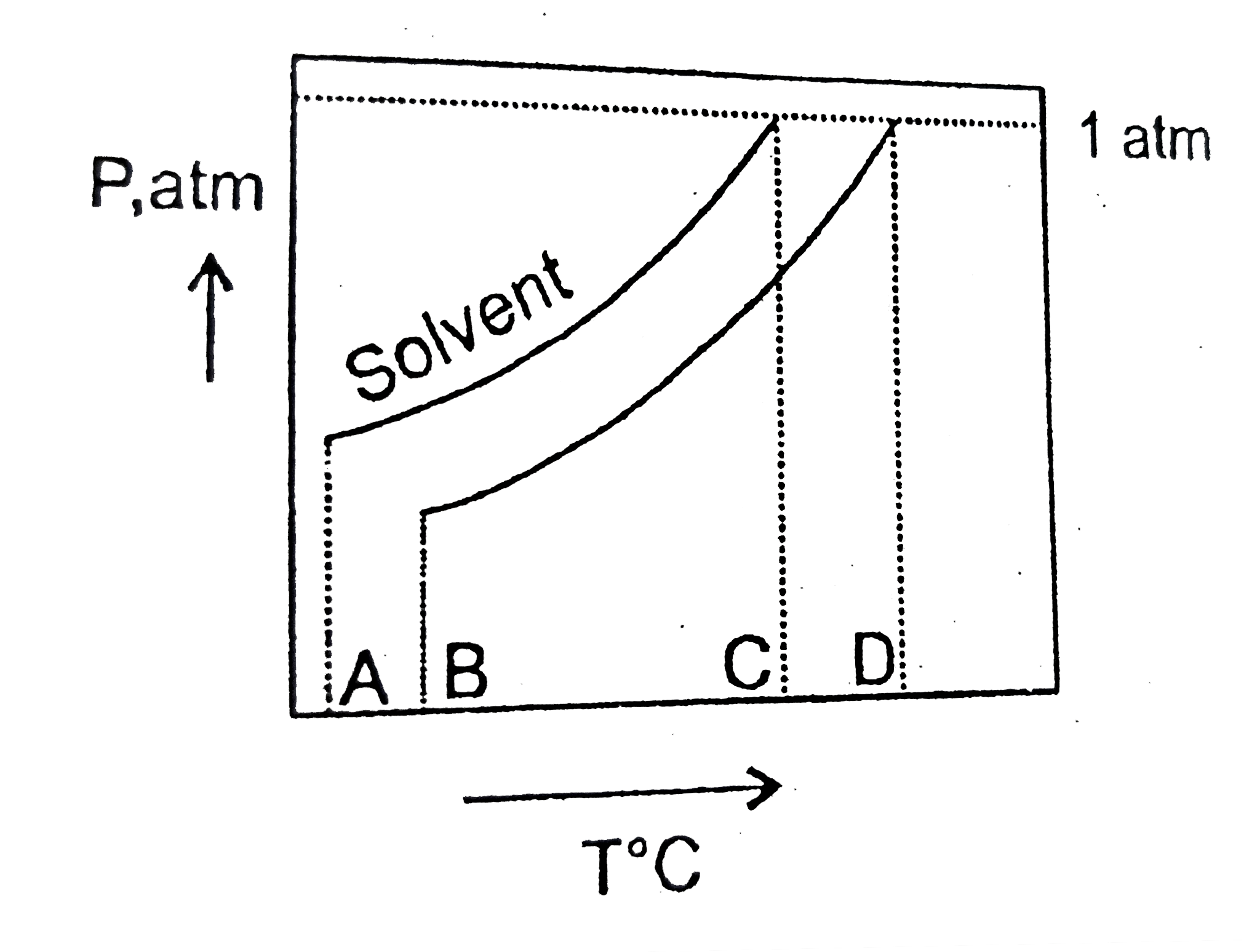 What Is The Normal Boiling Point Of The Solution Represented
What Is The Normal Boiling Point Of The Solution Represented
 Vapor Pressure 1atm 760 Mmhg 101 3kpa B Is A Gas B Is
Vapor Pressure 1atm 760 Mmhg 101 3kpa B Is A Gas B Is
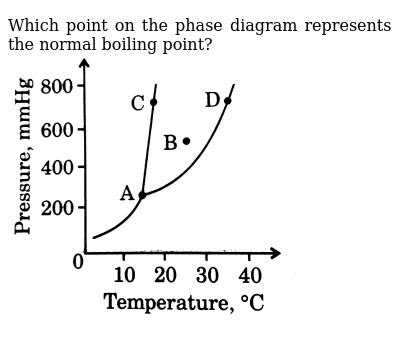 Which Kelvin Temperatures Represent Respectively The
Which Kelvin Temperatures Represent Respectively The
 How Do I Make A Phase Diagram For Water Socratic
How Do I Make A Phase Diagram For Water Socratic
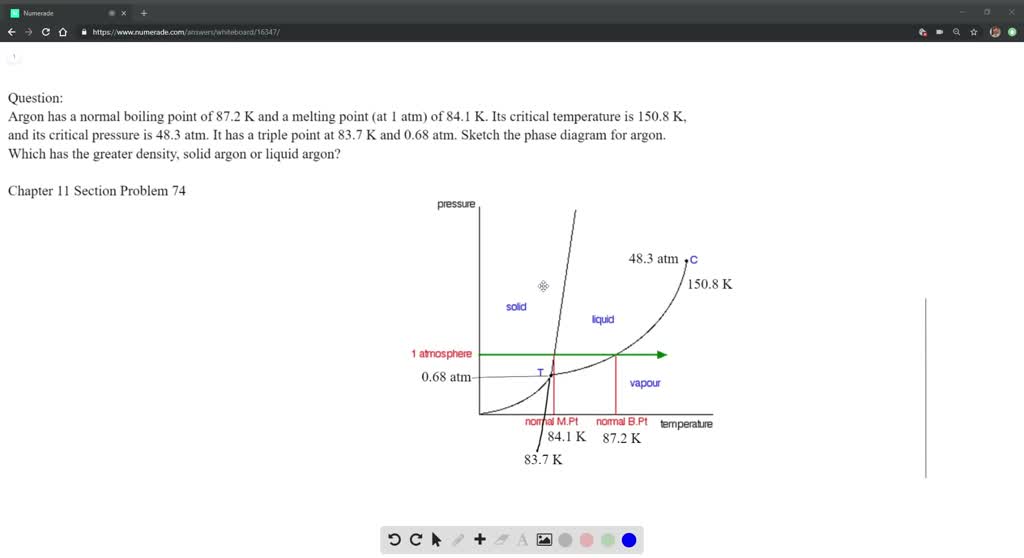 Solution For Nitrogen Has A Normal Boiling Point Of 77 3 K
Solution For Nitrogen Has A Normal Boiling Point Of 77 3 K
 Water Phase Diagram For Powerpoint Pslides
Water Phase Diagram For Powerpoint Pslides
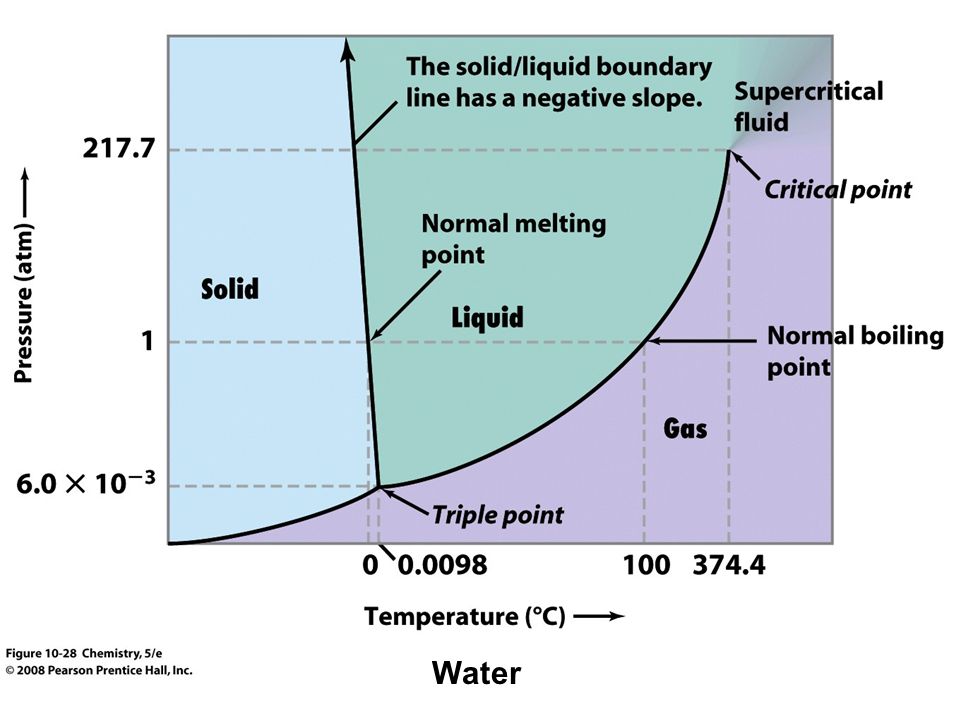 Liquids Solids And Phase Changes Ppt Video Online Download
Liquids Solids And Phase Changes Ppt Video Online Download
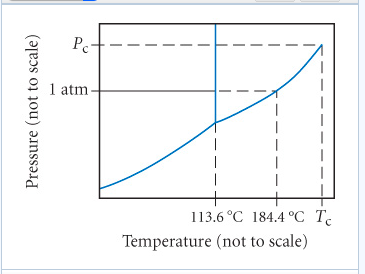 Solved Part A What Is The Normal Boiling Point For Iodine
Solved Part A What Is The Normal Boiling Point For Iodine
A 20 Liter Test Stand With Gas Purification For Liquid Argon

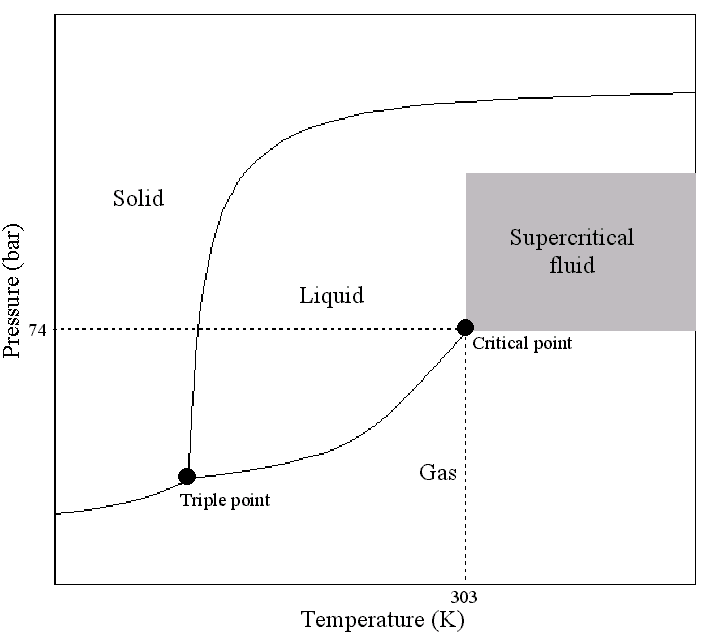



Belum ada Komentar untuk "Normal Boiling Point On Phase Diagram"
Posting Komentar