The Orbital Diagram For A Ground State Nitrogen Atom Is
Lowest available energy to some other higher energy orbital. The 1s orbital the 2s orbital and the 2p orbital.
Which ground state atom has an electron configuration described by the following orbital diagram.
The orbital diagram for a ground state nitrogen atom is. The electron configuration of a ground state co atom is 1b a ar4s24d7 b ar4s13d5 c 1s22s22p63s23d9 d ar4s23d7 e ne3s23d7. The reason for this is a result of the electron configuration. The orbital diagram for a ground state oxygen atom is a.
The orbital diagram for a ground state nitrogen atom is. An excited state means that typically the valence electron has moved from its ground state orbital ie. Experimentally it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.
The ground state electron configuration of p is ne3s23p3. C has two unpaired electrons in its ground state. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
Textbook solutions expert qa. In question 169 b there is a picture which shows the electron configuration for nitrogen. In this case the 2s and 2p orbitals are the.
Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. So any electron configuration in which the last electron again the valence electron is in a higher energy orbital this element is said to be in an excited state. This subshell is full.
A nitrogen atom has 3 orbitals. The remaining three electrons will go in the 2p orbital. Best answer 100 1 rating previous question next question get more help from chegg.
There are two arrows for the 1s orbital 2 arrows in the 2s orbital and one arrow in each of the three 2p orbitals. The orbital diagram for a ground state carbon atom is a. The 4p subshell contains three orbitals ml 1 0 1.
Answer to the orbital diagram for a ground state nitrogen atom is. By hunds rule the electron configuration of carbon which is 1s 2 2s 2 2p 2 is understood to correspond to the orbital diagram shown in c. The question asks us to determine whether the electron configuration represents the excited state or ground state for the atom.
The 3s subshell contains one orbital ml0 which holds two spin paired electrons. Nitrogen is the seventh element with a total of 7 electrons.
Electron Configuration And Chemical Periodicity
 Choose The Orbital Diagram That Represents The Ground
Choose The Orbital Diagram That Represents The Ground
 The Orbital Diagram For A Ground State Nitrogen Atom Is 1s
The Orbital Diagram For A Ground State Nitrogen Atom Is 1s
 What Is The Orbital Diagram For Nickel Quora
What Is The Orbital Diagram For Nickel Quora
 Nh3 Molecular Geometry Hybridization Bond Angle And
Nh3 Molecular Geometry Hybridization Bond Angle And
 Choose The Orbital Diagram That Represents The Ground State Of N
Choose The Orbital Diagram That Represents The Ground State Of N
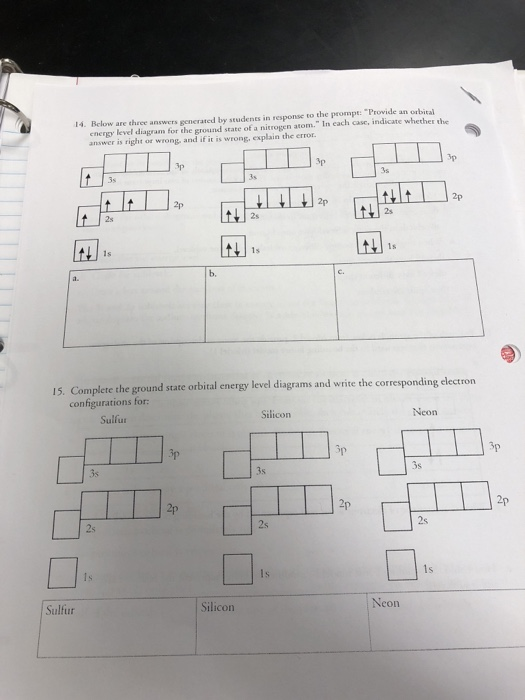 Solved Electron Configurations Pogil Unit 3 Assignment I
Solved Electron Configurations Pogil Unit 3 Assignment I
 Solution Choose The Orbital Diagram That Chemistry
Solution Choose The Orbital Diagram That Chemistry
Chem 1411 Study Guide For Test 3 Chapters 7 8 9 Pages 1
 Electron Configuration Orbital Diagram Nitrogen
Electron Configuration Orbital Diagram Nitrogen
 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
 Quantum Numbers Atomic Orbitals And Electron Configurations
Quantum Numbers Atomic Orbitals And Electron Configurations
Chapter 7 Quantum Theory And The Electronic Structure Of Atoms
 The Orbital Diagram For A Ground State Nitrogen Atom Is Page
The Orbital Diagram For A Ground State Nitrogen Atom Is Page
Chapter 7 Quantum Theory And The Electronic Structure Of Atoms
 Webelements Periodic Table Nitrogen Properties Of Free Atoms
Webelements Periodic Table Nitrogen Properties Of Free Atoms

Chem4kids Com Nitrogen Orbital And Bonding Info
 2 The Orbital Radius Difference Between The Ground State Of
2 The Orbital Radius Difference Between The Ground State Of
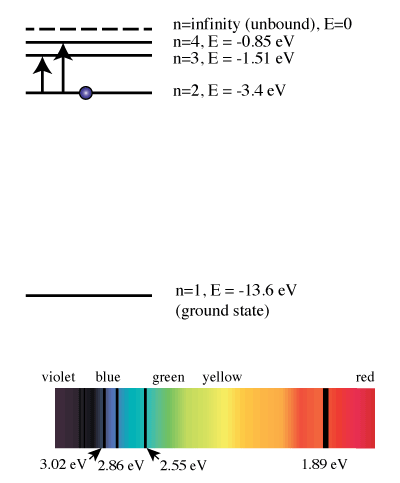


Belum ada Komentar untuk "The Orbital Diagram For A Ground State Nitrogen Atom Is"
Posting Komentar