Examine The Following Phase Diagram And Determine What Phases Exists At Point A
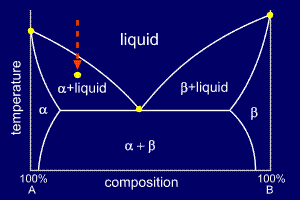
A bos has a lower density than bol. Examine the following phase diagram and determine what phase exists at point f.
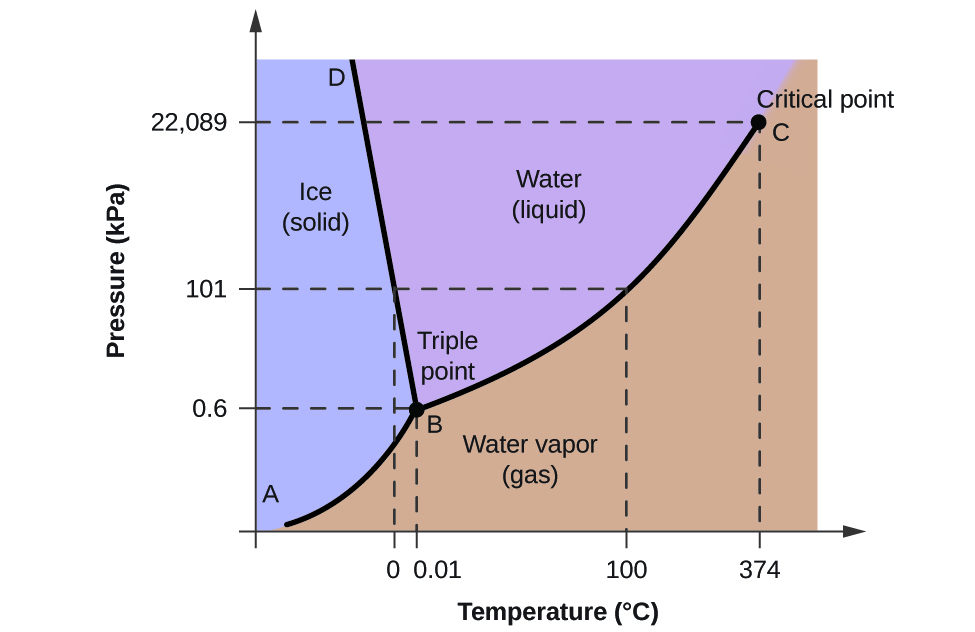
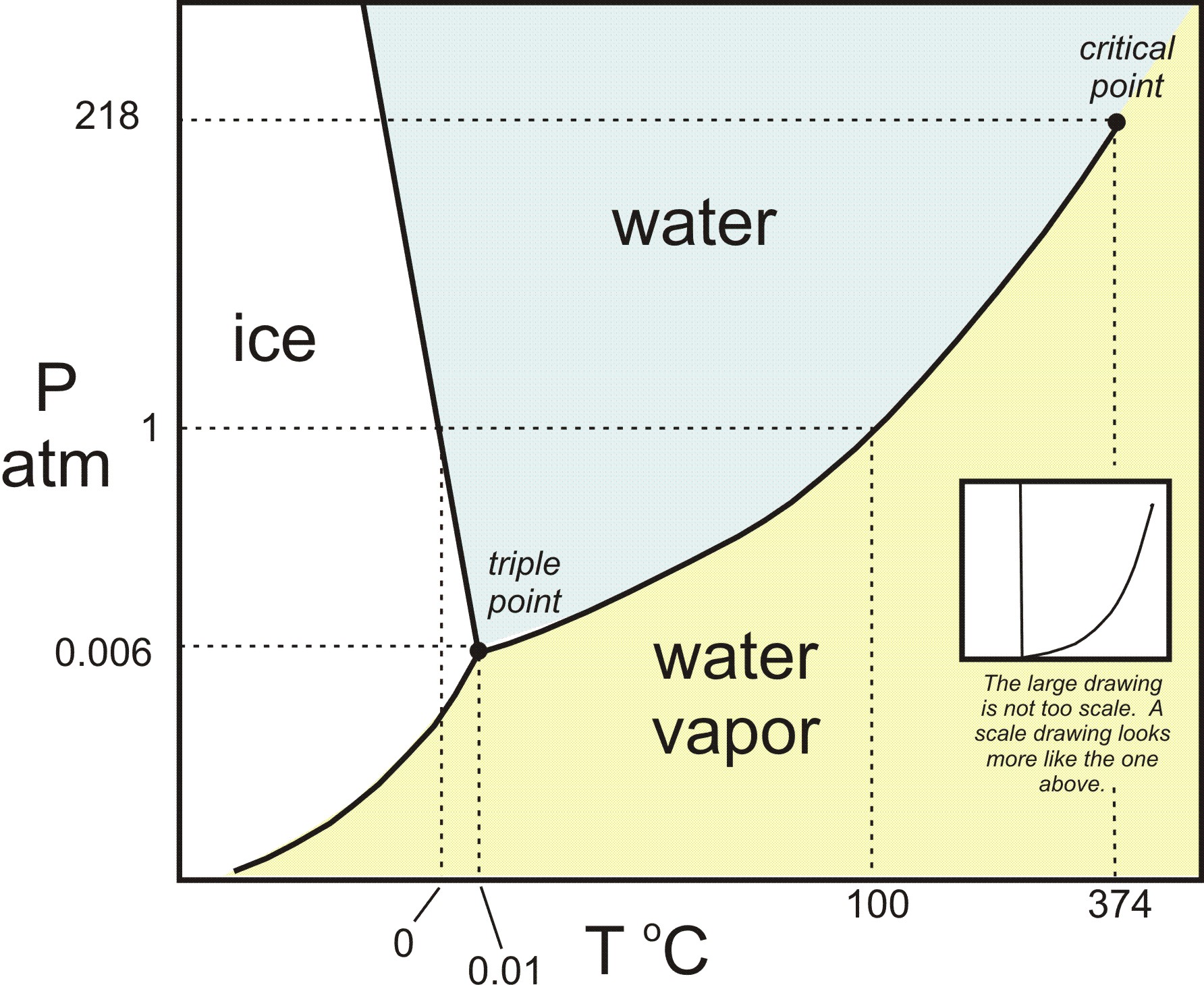
Phase diagrams are graphs of the relationship between the pressure and the temperature of a gas.

Examine the following phase diagram and determine what phases exists at point a. The negative slope of the sl equilibrium line means that increasing the pressure with s l moves the system into the liquid region. Increasing temperature with a phase change from solid to vapor examine the following phase diagram and determine what phase exists at point f. Neon atoms are attracted to each other by a dipole dipole forces.
A phase diagram has three curves representing the temperatures and corresponding pressures for the transitions from one state to another. The phase diagram with s solid l liquid and g gas is shown below. Aincreasing temperature with a phase change from solid to liquid bincreasing temperature with a phase change from solid to vapor cincreasing temperature with a phase change from liquid to vapor dincreasing temperature with no phase change eincreasing temperature beyond the critical point7.
Consider the following phase diagram and identify the process occurring as one goes from point c to point d. Chapter 12 consider the following phase diagram and identify the process occurring as one goes from point c to point d. Vapor which of the following intermolecular forces is the weakest.
Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first. Lets start by looking at a phase diagram and exploring everything thats on it. B london dispersion forces.
A vapor liquid. Sketch the phase diagram of water and explain how the above phenomenon manifests itself in the phase diagram. Examine the phase diagram for the substance bogusium bo and select the correct statement.
Ccl 4 cbr 4 c. These diagrams show the solid liquid and gas phases. B the triple point for bo is at a higher temperature than the melting point for bo.
A b temperature 760 torr f 18. C 7h 16 c 5h 12 b. Examine the following phase diagram and identify the feature represented by point a and point b.
H 2o h 2s. Phase diagrams are unique to every different substance. Examine the following phase diagram and determine what phase exists at point 760 som temperature a supercritical fluid b liquid c vaporliquid d vapor e solid the phase diagram of a substance i s given below.
Examine the following phase diagram and determine what phase exists at point f. Ammonias unusually high melting point is the result of 20. A phase diagram is a graph of the physical state of a substance solid liquid or gas and the temperature and pressure of the substance.
C bo changes from a solid to a liquid as one follows the line from c to d. The x axis of the graph shows temperature. Examine the following phase diagram and determine what phase exists at point f.
Phase Change Evaporation Condensation Freezing Melting
 Selected Answer B 444 G Ar Correct Answer B 444 G Ar
Selected Answer B 444 G Ar Correct Answer B 444 G Ar
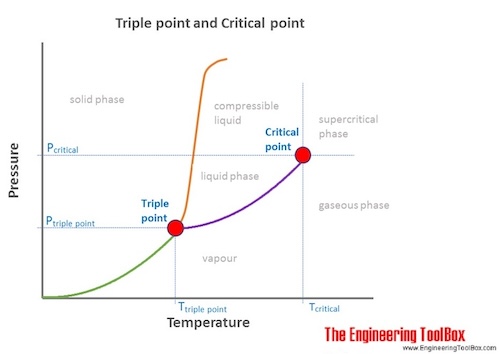 Critical Temperatures And Pressures For Some Common Substances
Critical Temperatures And Pressures For Some Common Substances

 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
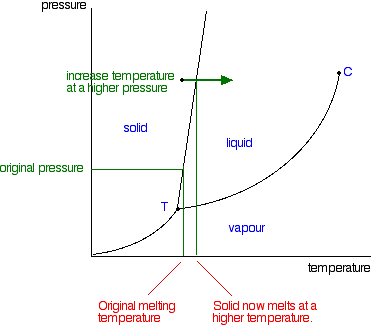 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
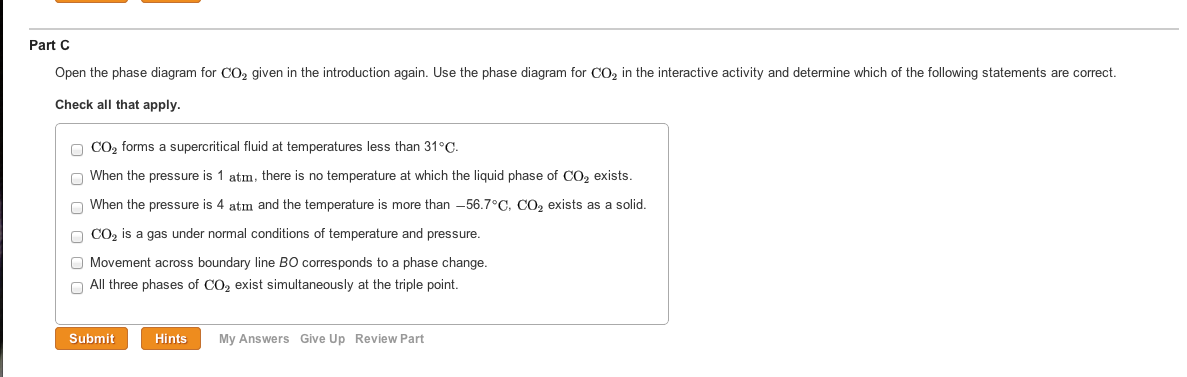 Open The Phase Diagram For Co2 Given In The Introd
Open The Phase Diagram For Co2 Given In The Introd
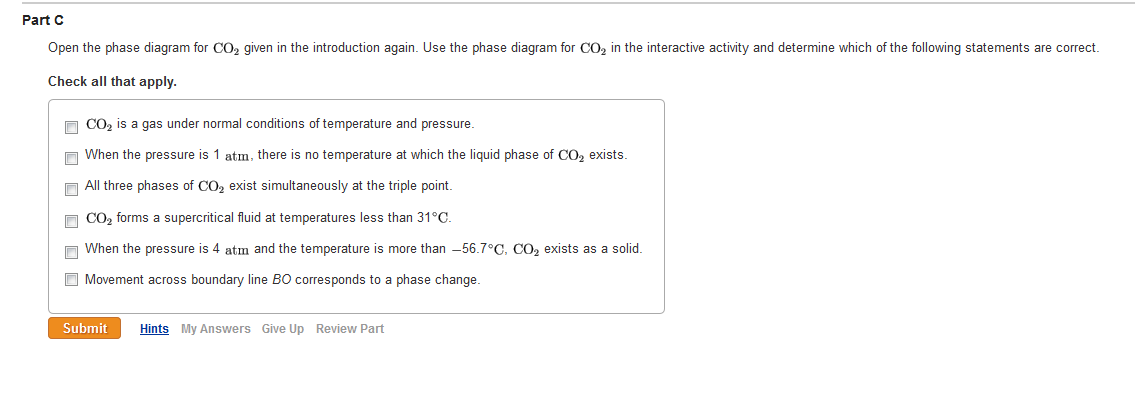 Solved Open The Phase Diagram For Co2 Given In The Introd
Solved Open The Phase Diagram For Co2 Given In The Introd
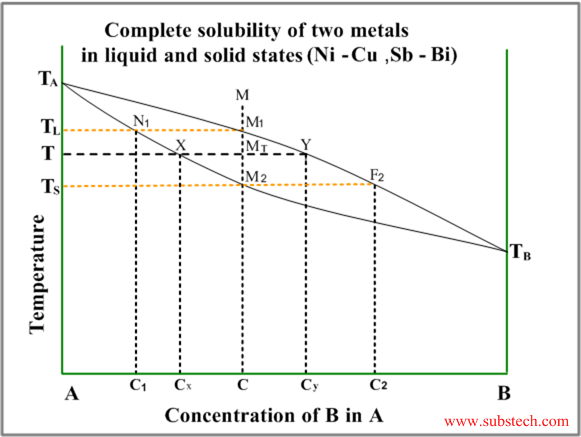 Phase Transformations And Phase Diagrams Substech
Phase Transformations And Phase Diagrams Substech
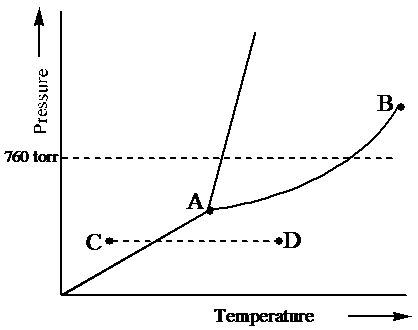 Solved Examine The Following Phase Diagram And Determine
Solved Examine The Following Phase Diagram And Determine
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
/phase-changes-56a12ddd3df78cf772682e07.png) List Of Phase Changes Between States Of Matter
List Of Phase Changes Between States Of Matter
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
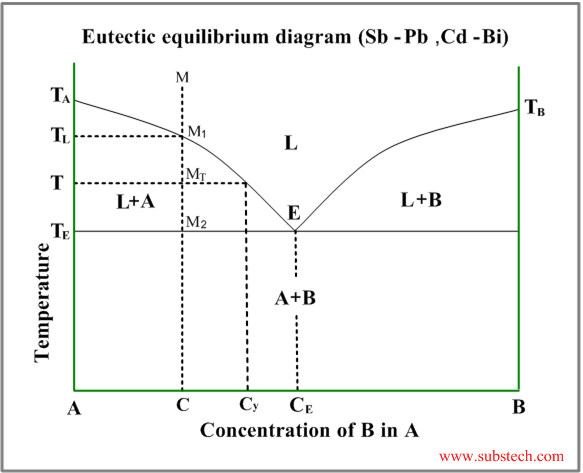 Phase Transformations And Phase Diagrams Substech
Phase Transformations And Phase Diagrams Substech
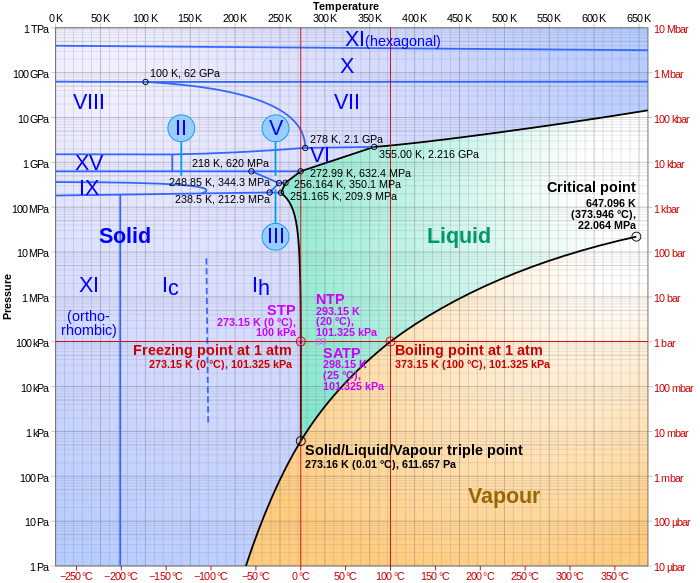
 Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point
Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point



Belum ada Komentar untuk "Examine The Following Phase Diagram And Determine What Phases Exists At Point A"
Posting Komentar