Click On The Point Of The Energy Diagram That Represents The Activated Complex Transition State
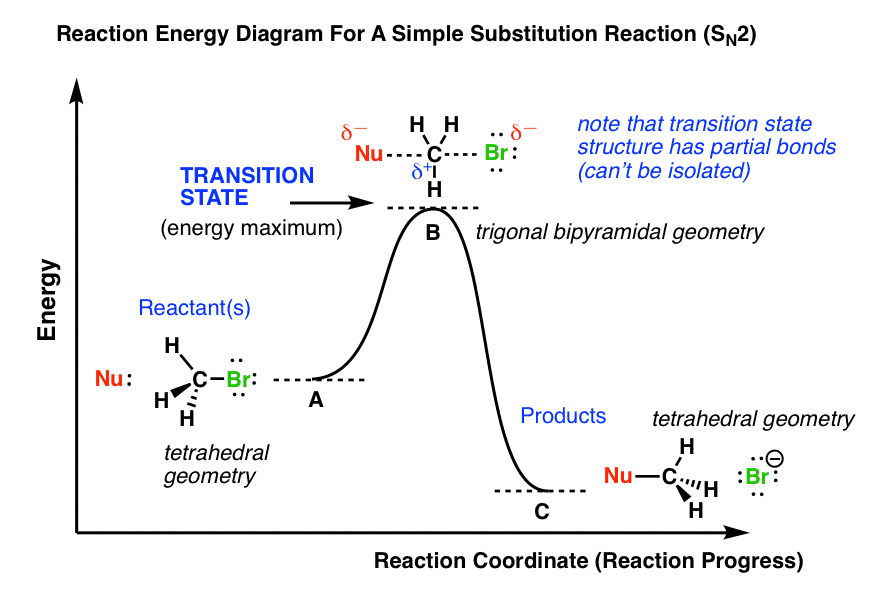
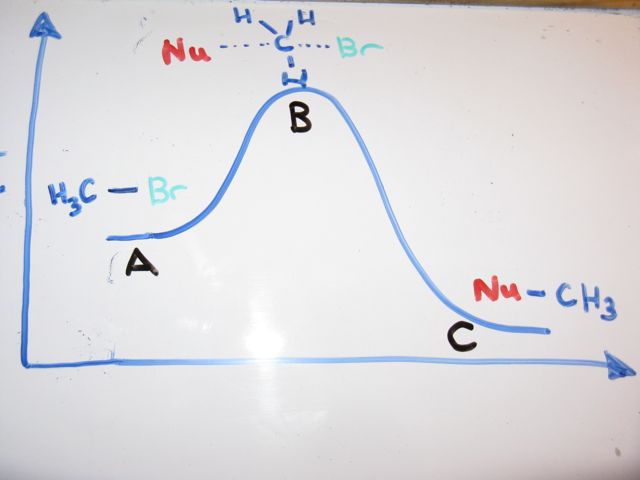
At the very top of the energy barrier the reaction is at its transition state ts which is the point at which the bonds are in the process of breaking and forming. Answer to on the energy diagram that represents the activated complex transition state identify the point answer bank activated.
 Activation Energy And Temperature Dependence Boundless
Activation Energy And Temperature Dependence Boundless
Answer to label this diagram.

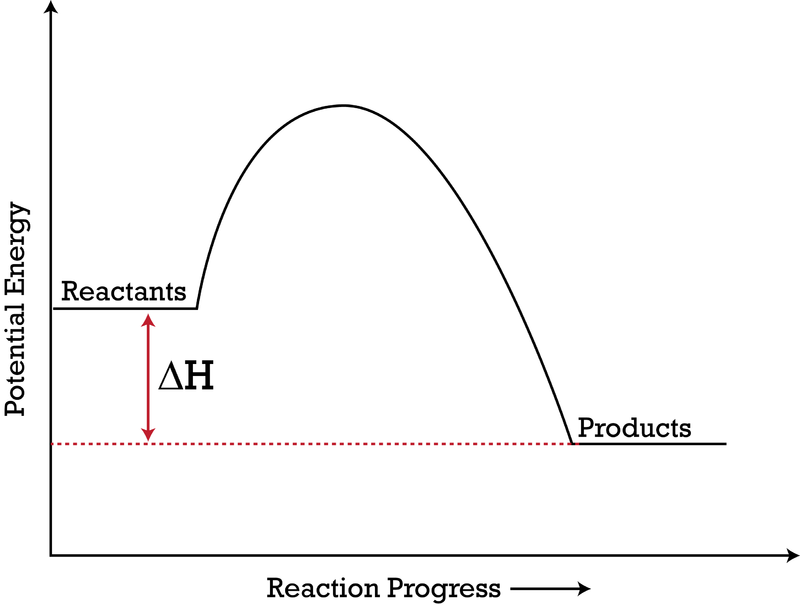
Click on the point of the energy diagram that represents the activated complex transition state. An activated complex is an intermediate state that is formed during the conversion of reactants into products. A transient and dynamic state that unlike more stable species does not have any definable lifetime. The transition state is an activated complex.
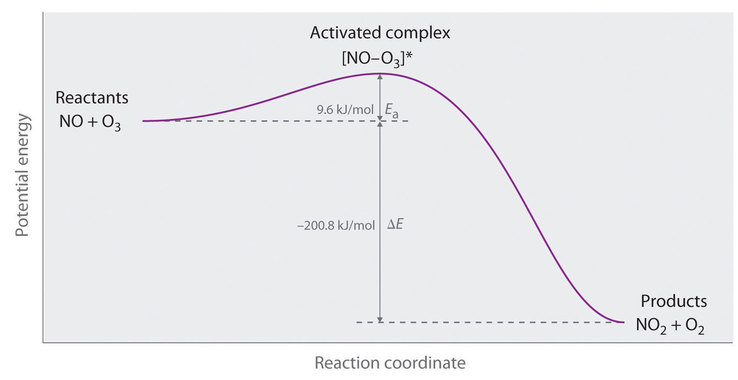
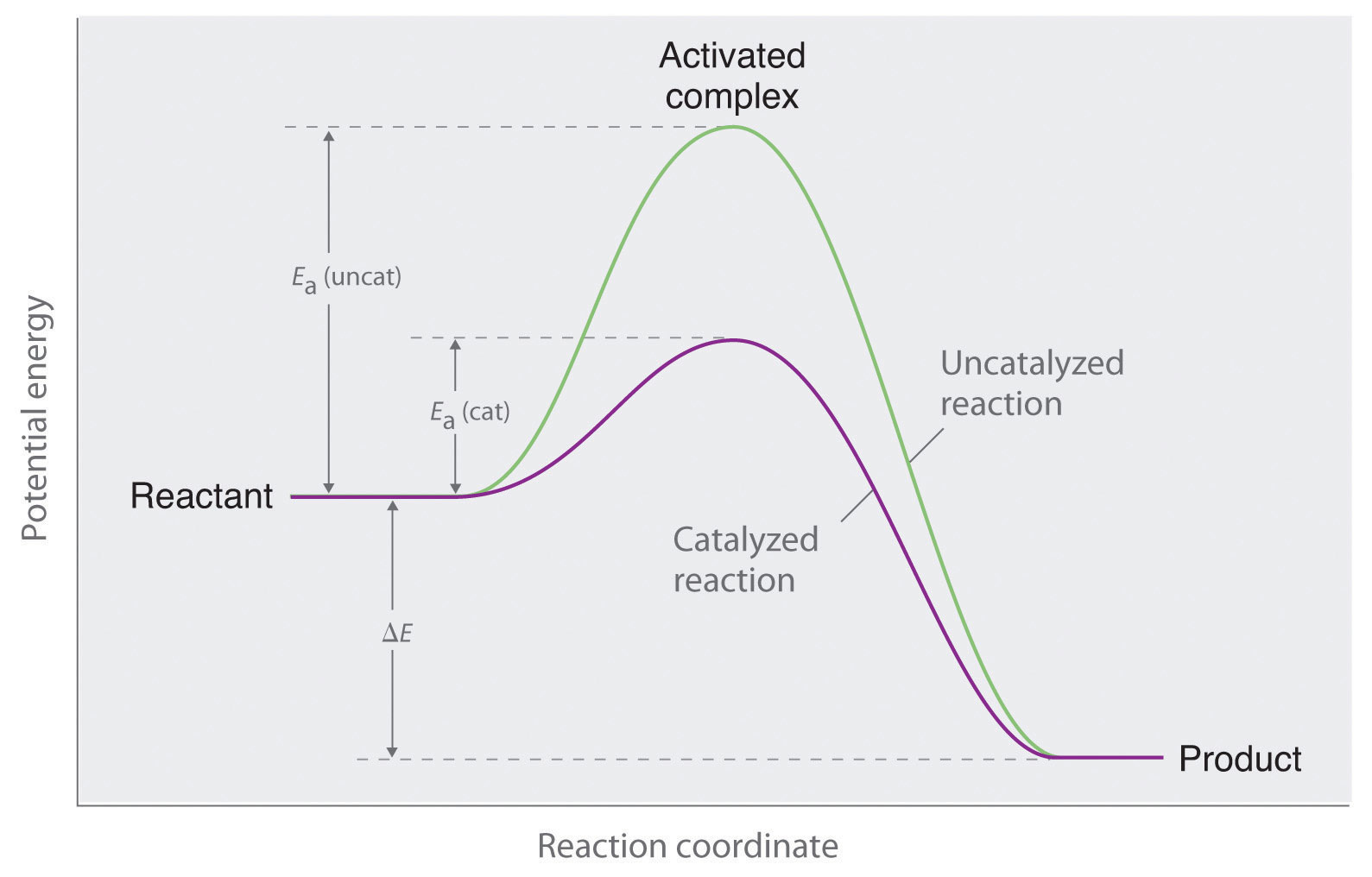
The energy of the activated complex if the reaction is exothermic describe the positions of the reactants and products on a potential energy graph. Activated complex an intermediate structure formed in the conversion of reactants to products. Which curve represents the cat.
The potential energy of an activated complex is typically greater than the potential energy of the reactants and products. The activation energy of a chemical reaction is the difference between the energy of the activated complex and the energy of the reactants. A transient and dynamic state that unlike more stable species does not have any definable lifetime.
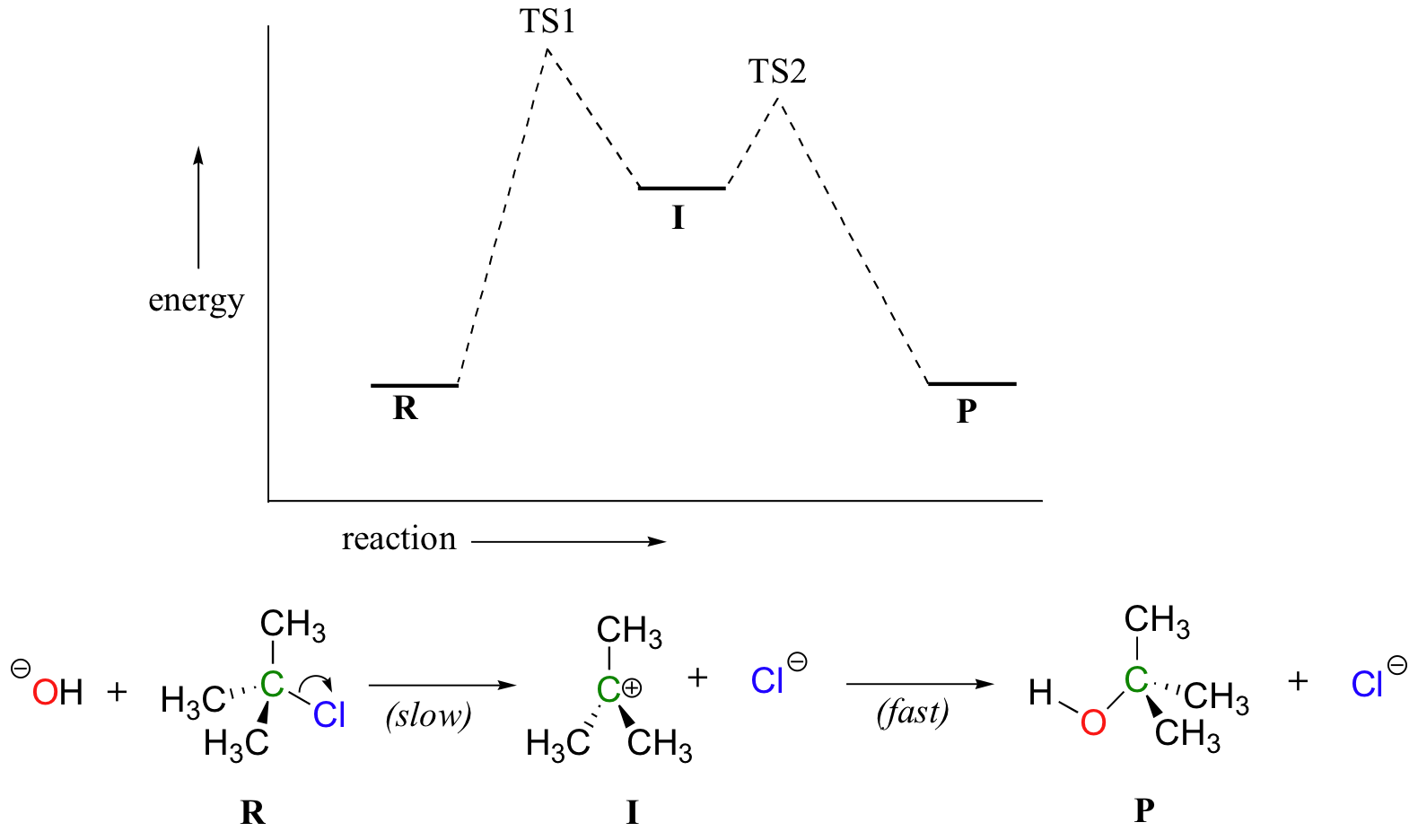
This state is also known as an activated complex. However it differs from the transition state in that the transition state represents only the highest potential energy configuration of the atoms during the reaction while the activated complex refers to a range. The activated complex is often confused with the transition state and is used interchangeably in many textbooks.
It is the structure at the maximum energy point in the pe diagram. What does the highest point of a potential energy diagram indicate. That is because some of the energy is already used.
At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Note that a transition state is also known as an activated complex.
At the very top of the energy barrier the reaction is at its transition state ts which is the point at which the bonds are in the process of breaking and forming. The transition state is an activated complex. An activated complex is the structure that results in the maximum energy point along the reaction path.
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
 Collision Theory Use The Collision Theory To Explain The
Collision Theory Use The Collision Theory To Explain The
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
 How Can I Draw A Simple Energy Profile For An Endothermic
How Can I Draw A Simple Energy Profile For An Endothermic
 Collision Theory Use The Collision Theory To Explain The
Collision Theory Use The Collision Theory To Explain The
Hammond Postulate Polanyi Hammond Postulate Chemgapedia
 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
Solved Consider The Reaction A B C The Diagram Belo
 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
 Reaction Mechanisms And Potential Energy Diagrams Ck 12
Reaction Mechanisms And Potential Energy Diagrams Ck 12
 14 5 Microscopic View Of Reaction Rates Chemistry Libretexts
14 5 Microscopic View Of Reaction Rates Chemistry Libretexts
 Physical Chemistry Transition State And Free Energy
Physical Chemistry Transition State And Free Energy
 Reaction Mechanism Britannica Com
Reaction Mechanism Britannica Com
 Enzymatic Transition States And Transition State Analogues
Enzymatic Transition States And Transition State Analogues
Ch103 Chapter 7 Chemical Reactions In Biological Systems
 What Is The Activation Energy For A Reverse Reaction Quora
What Is The Activation Energy For A Reverse Reaction Quora
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia



Belum ada Komentar untuk "Click On The Point Of The Energy Diagram That Represents The Activated Complex Transition State"
Posting Komentar