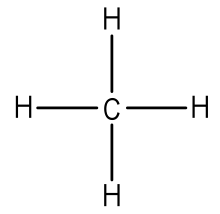
Electron Dot Diagram For Methane
We also have a handy video on the 5 things you need to know for general chemistry. Methane ch4 1 methane ch4.
 Electron Dot Structure Of Alkane Alkene Alkyne Education
Electron Dot Structure Of Alkane Alkene Alkyne Education
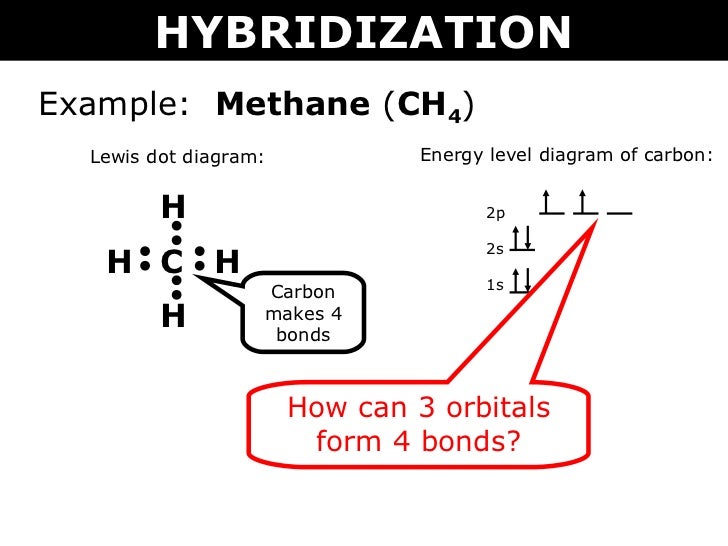
Carbon typically like to make 4 bonds because carbon has 4 valence e and it wants to get 8 total making four bonds will give it 8 total.

Electron dot diagram for methane. We show two ways to draw the ch4 lewis structure methane. However the carbonate anion co32 does have a lewis dot structure. Covalent bonding guidelines for non ions.
It is an ionic compound so it would not have a lewis dot structure. The ch 4 lewis structure is one of the most frequently tested lewis structures. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell.
Methane is a one carbon compound in which the carbon is attached by single bonds to four hydrogen atomsit is a colourless odourless non toxic but flammable gas bp. Carbon has 4 and each h has 1 total 4. Ch4 has 8 total valence electrons.
We show two ways to draw the ch4 lewis structure methane. Review of lewis dot and valence electrons. Toggle navigation biochemhelp blog more help for you.
A step by step explanation of how to draw the ch4 lewis dot structure. The lewis dot structure for ch4 is shown above. This is because they can share a maximum of two electrons.
First count total number of valence electrons. Each atom in the bond has a full valence with carbon having access to eight electrons and each hydrogen having access to two this is why hydrogen only needs twothe covalent bonds between the c and the h are similar to the ones formed between two hs. For ch 4 you have a total of 8 total valence electrons.
Practicing calculating formal charges. Explains how to draw the lewis dot structure for ch 4 methane. Note that hydrogen atoms always go on the outside of a lewis dot structure.
Covalent bonding with electron dot formulas or lewis structures. We also have a handy video on the 5 things you need to know for general chemistry. Drawing the lewis structure for ch 4.
Methane for the ch4 lewis structure calculate the total number of valence electrons for the ch4 molecule ch4 has 8. Electrons are placed up to two on each side of the elemental symbol for a maximum of eight which is the number of electrons in a filled s and p shell. Electron dot structure valence electrons are represented by dots placed around the chemical symbol.
These kinds of structures can also be shown by representing each of the bonds with two dots. It has a role as a fossil fuel a member of greenhouse gas and a bacterial metabolite. Drawing the lewis structure for ch 4 named methane requires only single bondsits one of the easier lewis structures to draw.
 Ch4 Lewis Structure W Free Video Guide
Ch4 Lewis Structure W Free Video Guide
 Questions And Answers Cbse Icse Solutions Cbse Icse Study
Questions And Answers Cbse Icse Solutions Cbse Icse Study
:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png) Lewis Dot Structure Example Octet Rule Exception
Lewis Dot Structure Example Octet Rule Exception
 Lewis Dot Symbols And Lewis Structures Boundless Chemistry
Lewis Dot Symbols And Lewis Structures Boundless Chemistry
 How To Draw The Lewis Structure For C3h8 Propane
How To Draw The Lewis Structure For C3h8 Propane
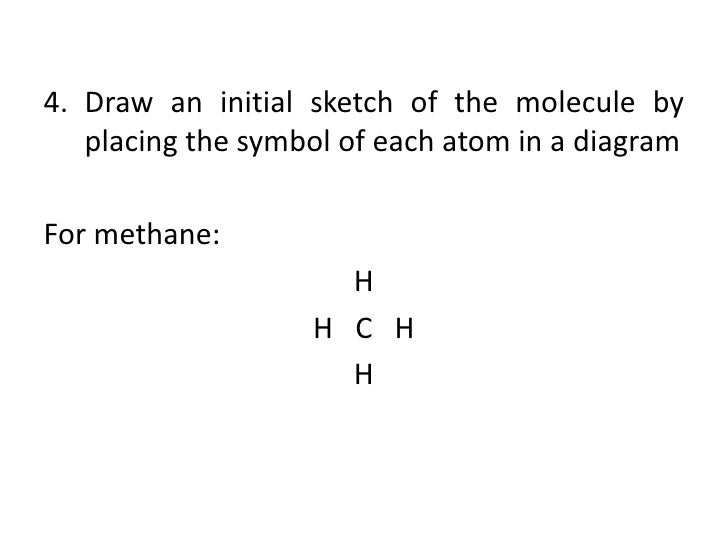 How To Draw Methane Ch4 Lewis Structure
How To Draw Methane Ch4 Lewis Structure
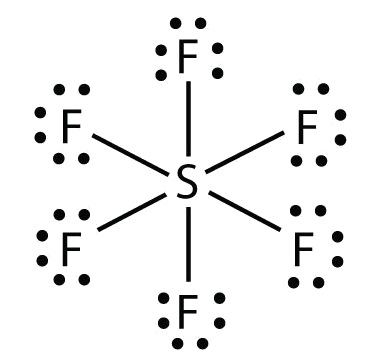 10 5 Writing Lewis Structures For Covalent Compounds
10 5 Writing Lewis Structures For Covalent Compounds
 Wedge And Dash Projection Definition And Example
Wedge And Dash Projection Definition And Example
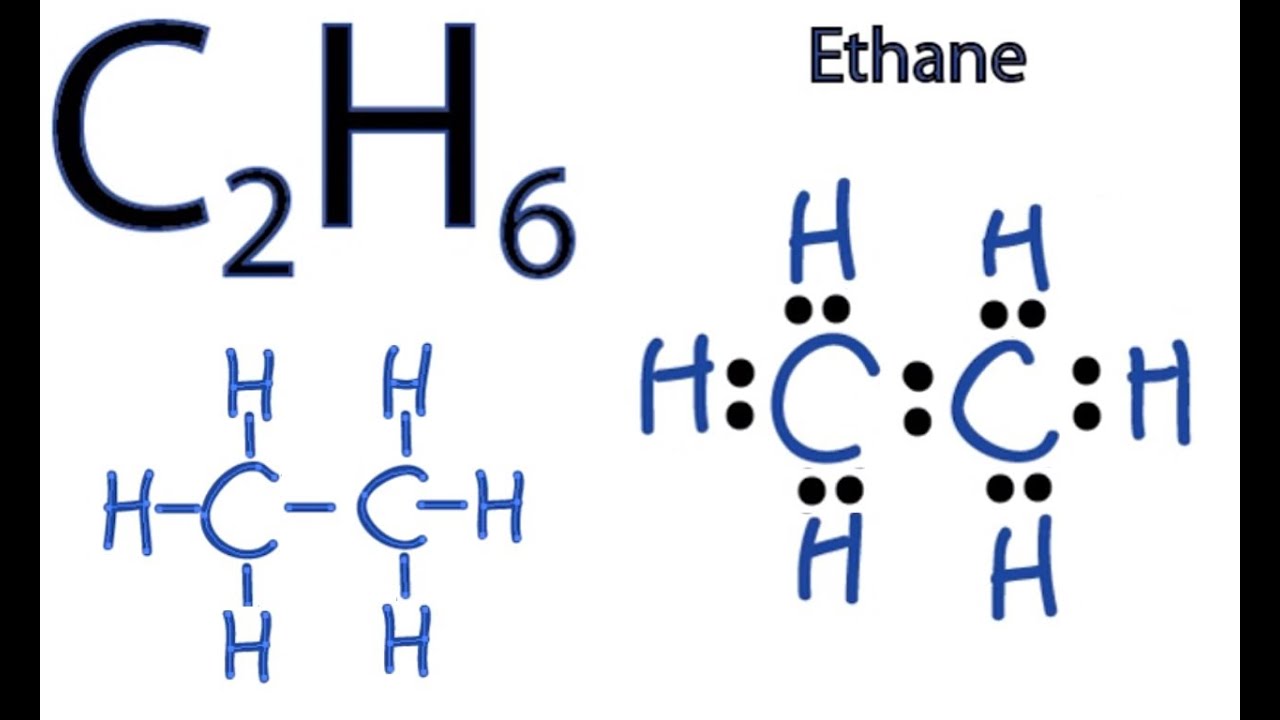 C2h6 Lewis Structure How To Draw The Dot Structure For C2h6
C2h6 Lewis Structure How To Draw The Dot Structure For C2h6
 How To Draw Methane Ch4 Lewis Structure
How To Draw Methane Ch4 Lewis Structure
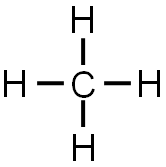 Covalent Bonding Electron Dot Diagrams Texas Gateway
Covalent Bonding Electron Dot Diagrams Texas Gateway
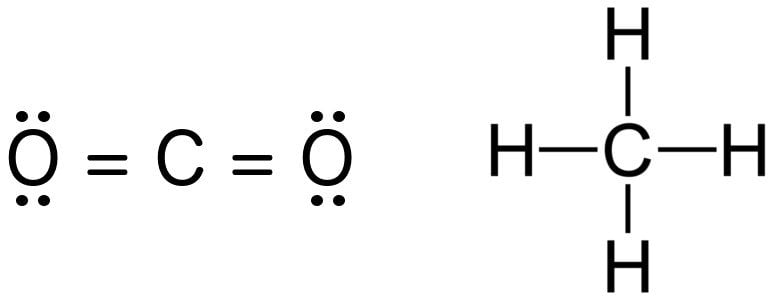 Bond Order Definition Calculation And Significance
Bond Order Definition Calculation And Significance
 What Is The Lewis Structure Of Methane Study Com
What Is The Lewis Structure Of Methane Study Com







Belum ada Komentar untuk "Electron Dot Diagram For Methane"
Posting Komentar