Be2 Molecular Orbital Diagram
A draw the molecular orbital diagram. For the second period elements the 2s and 2p orbitals are important for mo considerations.

The electronic configuration of bez 4 is4 be 1s2 2s1be2 molecule is formed by the overlap of atomic orbitals of both beryllium atomsnumber of valence electrons in be atom 2thus in the formation of be2 molecule two outer electrons of each be atom ie.

Be2 molecular orbital diagram. Bonding order is 0 meaning it does not bond and it is diamagnetic. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of.
D write the electron configuration of the ion. Well as the first point it is to be noted that an atom forms a molecule in order to get stabilised. N2 2 o2 2 be2 2 c2 explain please.
Molecular orbital diagrams of diatomic molecules introduction. Now we know that. A linear combination of properly oriented atomic orbitals for the formation of sigma s and pi p bonds.
C would this ion exist. For the ion be2. How to draw a molecular orbital for be2 ion.
Molecular orbital diagram for beryllium dimer be2 fill from the bottom up with 4 electrons total. Which of the following ions would be diamagnetic. How do you draw molecular orbitals.
In chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule. Indicate theirnumbers of unpaired electron and mention their magnetic propertiescalculate their bond orders and state which species is moststable. In other word we can say that in order to form a molecule the energies of the atomic orbitals should be lowered in the molecule.
Sketch the molecular orbitals of the h2 ion and draw its energy level diagram. 4 in all have to be accommodated in various molecular orbitals in the increasing order of their energiesthe. Draw the molecular orbital energy level diagram for each of the following species be2 be2 and be2.
The same method can be applied to other diatomic molecules but involving more than the 1s atomic orbitals. B calculate the bond order. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules.
The Nature Of The Chemical Bond In Be2 Be2 Be2 And Be3
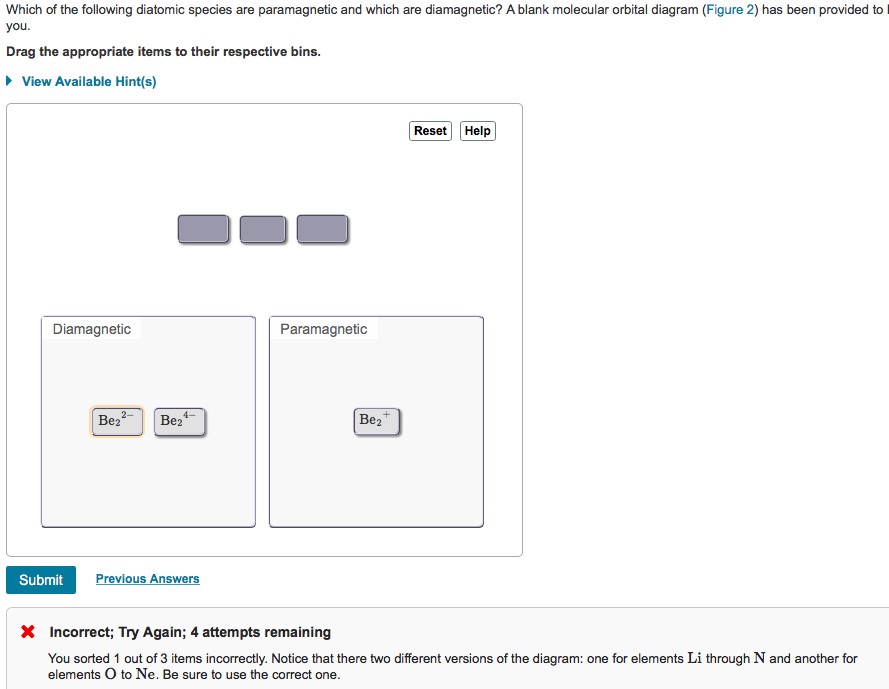 Solved Which O The Following Diatomic Species Are Paramag
Solved Which O The Following Diatomic Species Are Paramag
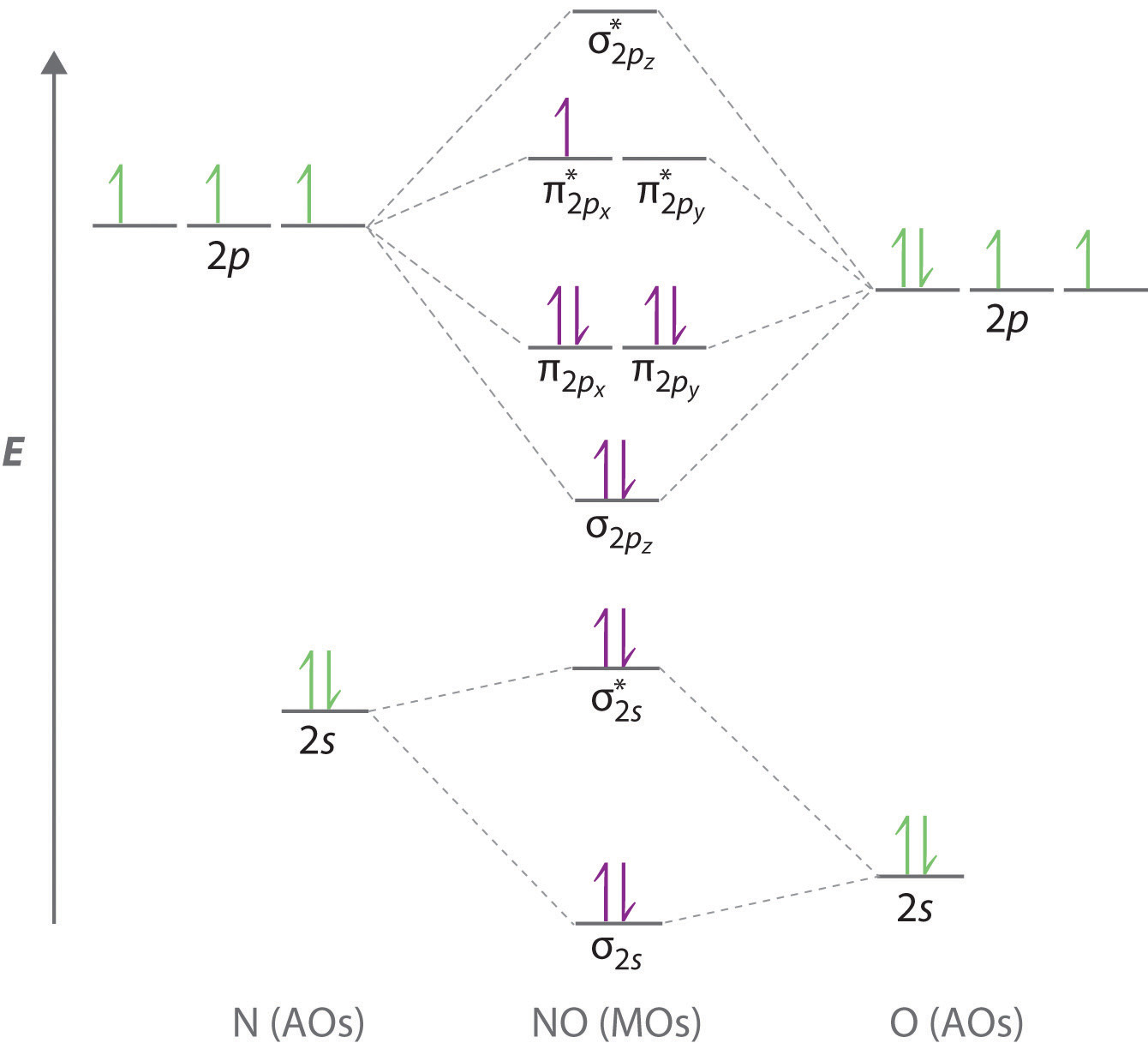 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts
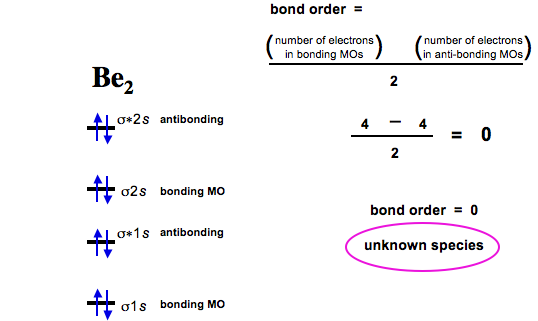 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
Using The Molecular Orbital Theory Why Does A Be2 Molecule
Molecular Orbital Approach To Bonding
 Use Mo Diagrams And The Bond Orders You Obtain From Them To
Use Mo Diagrams And The Bond Orders You Obtain From Them To
 Solution Given The Molecular Orbital Diag Clutch Prep
Solution Given The Molecular Orbital Diag Clutch Prep
The Nature Of The Chemical Bond In Be2 Be2 Be2 And Be3
Research Highlights J C Bose Fellowship
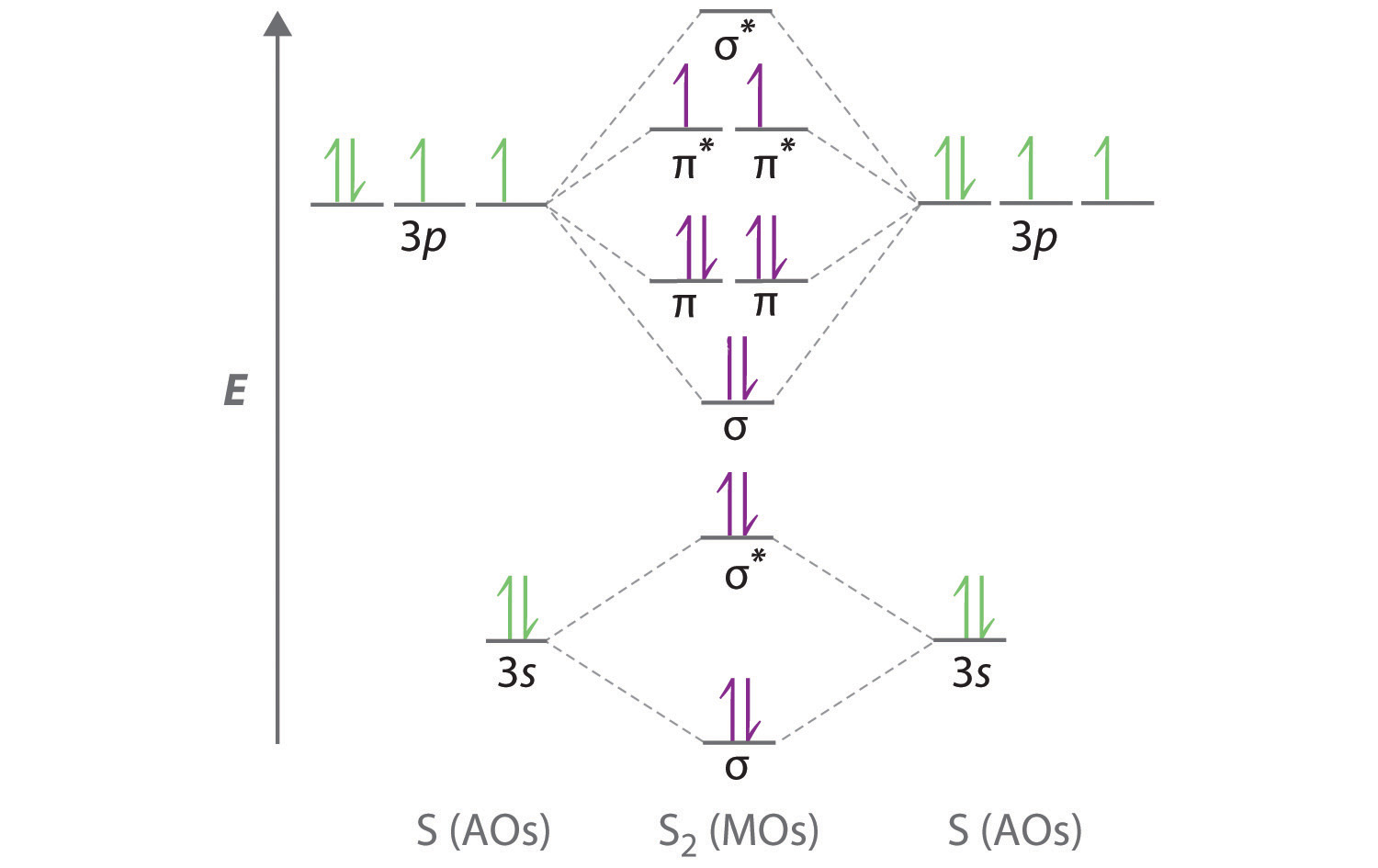 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts
 According To The Molecular Orbital Theory What Is The Bond
According To The Molecular Orbital Theory What Is The Bond
 Coupled Cluster And Quantum Monte Carlo Potential Energy
Coupled Cluster And Quantum Monte Carlo Potential Energy
 Openstax General Chemistry Ch8 Advanced Theories Of
Openstax General Chemistry Ch8 Advanced Theories Of
 Let S Focus On Valence Bond Theory Ppt Download
Let S Focus On Valence Bond Theory Ppt Download
 Videos Matching Molecular Orbital Theory Build Be2 Revolvy
Videos Matching Molecular Orbital Theory Build Be2 Revolvy
 Inorganic Chemistry What Are The Molecular Orbitals Shaped
Inorganic Chemistry What Are The Molecular Orbitals Shaped
 Molecular Structure Qtaim And Bonding Character Of Cation P
Molecular Structure Qtaim And Bonding Character Of Cation P
 Solved Use Mo Diagrams And The Bond Orders You Obtain From
Solved Use Mo Diagrams And The Bond Orders You Obtain From
 Use The Molecular Orbital Diagram Shown Below To Determine Which Of The Following Diatomic Species Has The Highest Bond Order A F22 B F22 C O22 D F2 E Ne22
Use The Molecular Orbital Diagram Shown Below To Determine Which Of The Following Diatomic Species Has The Highest Bond Order A F22 B F22 C O22 D F2 E Ne22
 The Nature Of The Chemical Bond In Be2 Be2 Be2 And Be3
The Nature Of The Chemical Bond In Be2 Be2 Be2 And Be3


Belum ada Komentar untuk "Be2 Molecular Orbital Diagram"
Posting Komentar