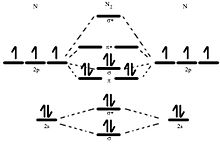
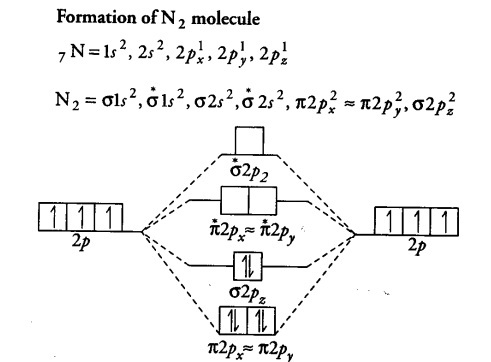
N2 2 Molecular Orbital Diagram
σ2 1sσ21sσ22sσ22sπ22pπ22p n2 14 e. Draw the molecular orbital diagram for n2 ion and calculate the bond order.
 Bonding In Homonuclear Diatomic Molecules O2 O2 O2 2
Bonding In Homonuclear Diatomic Molecules O2 O2 O2 2
First though notice that the p orbitals are supposed to be degenerate.
N2 2 molecular orbital diagram. Draw the molecular orbital diagram for n 2 ion and calculate the bond order. If we build the mo diagram for n2 it looks like this. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of.
Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. G means gerade or even symmetry upon inversion and u means ungerade or odd symmetry upon inversion.
Anyways for the electron configurations you would use a notation like the above. σ2 1sσ21sσ22sσ22sπ22pπ22pσ22pπ12pπ12p b bond orders are. The 2p x orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane.
σ2 1sσ21sσ22sσ22sπ22pπ22pσ22pπ12p n2 2 16 e. σ2 1sσ21sσ22sσ22sπ22pπ22pσ12p n2 212 e. Molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you get sigma2s2sigma2s2pi2p4sigma2p2.
They werent drawn that way on this diagram but they should be. Answers to molecular orbitals problem set 1. In both molecules the pi symmetry molecular orbitals are the same.
Here is the full molecular orbital diagram for n 2. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. Indicate if it is diamagnetic or paramagnetic.
σ2 1sσ21sσ22sσ22sπ22pπ22pσ22p n2 15 e. Indicate if it is diamagnetic or paramagnetic. A n2 13 e.
Summary mo theory lcao mo theory is a simple method for predicting the approximate electronic structure of molecules. Perpendicular to these in the yz plane the 2p y orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital. Bond order is 3 and it is paramagnetic.
Next well see that symmetry will help us treat larger molecules in.
Molecular Nitrogen And Related Diatomic Molecules
 Solved Write The Electron Configuration For The First
Solved Write The Electron Configuration For The First
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
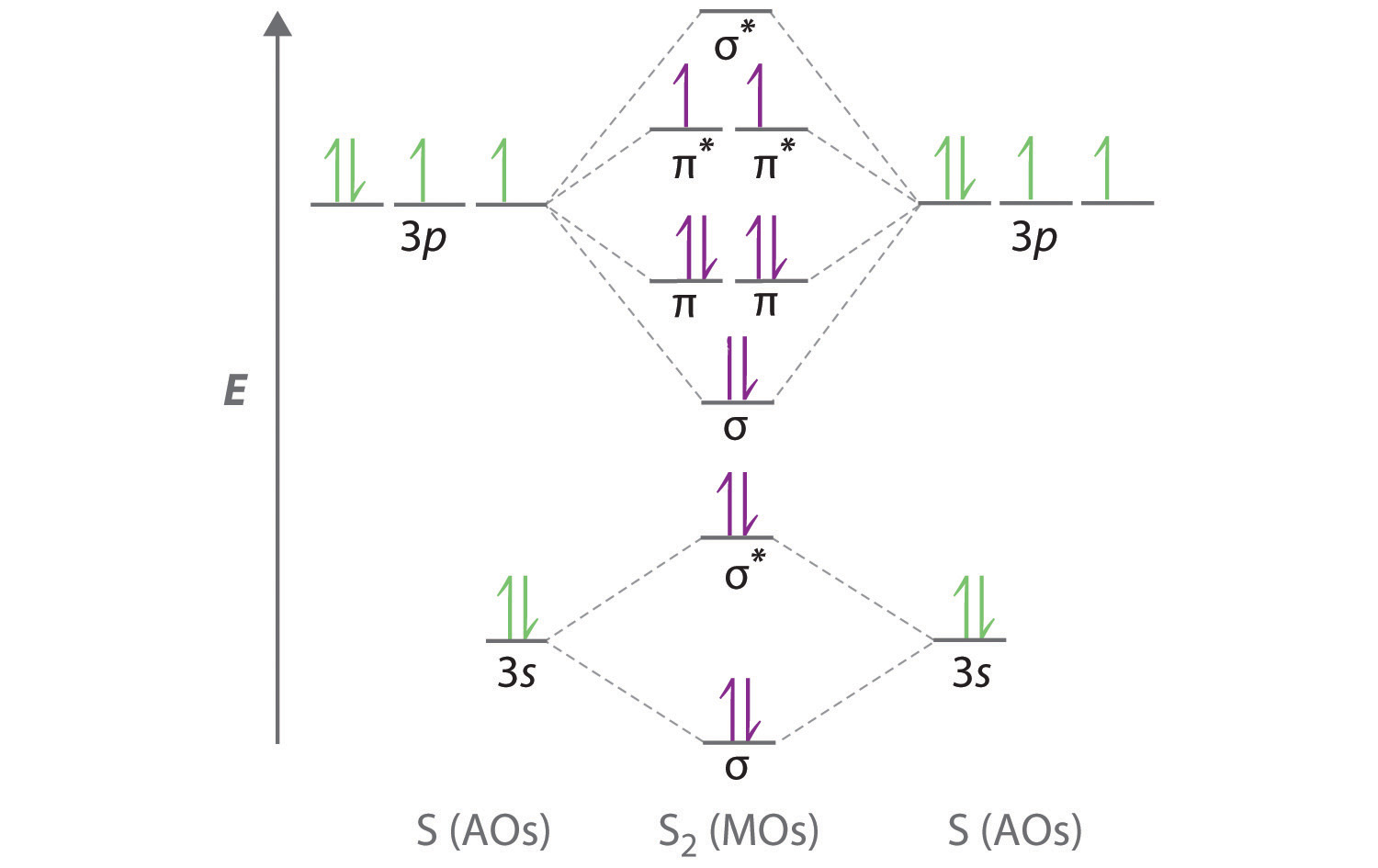 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
 Draw The Molecular Orbital Diagram Of O2 Or N2 Brainly In
Draw The Molecular Orbital Diagram Of O2 Or N2 Brainly In
 Solved Consider The Molecule N22 1 How Many Valence El
Solved Consider The Molecule N22 1 How Many Valence El
 What Is Mo Theory And How To Draw Mo Diagram For Different
What Is Mo Theory And How To Draw Mo Diagram For Different
Molecular Orbital Energy Level Diagrams Hydrogen
Section 5 Observation 3 Ionization Energies Of Diatomic

 Use The Molecular Orbital Energy Level Diagram To Show That
Use The Molecular Orbital Energy Level Diagram To Show That
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 Which Of The Following Is Diamagnetic 1 N2 2 N22 3
Which Of The Following Is Diamagnetic 1 N2 2 N22 3
 By Writing Molecular Orbital Configuration For No Co O2
By Writing Molecular Orbital Configuration For No Co O2
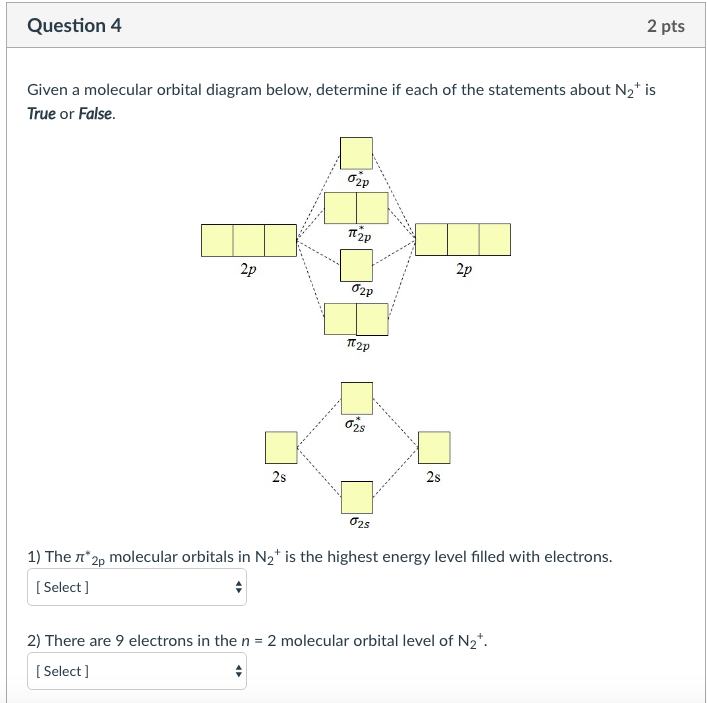 Solved 2 Pts Question 4 Given A Molecular Orbital Diagram
Solved 2 Pts Question 4 Given A Molecular Orbital Diagram
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
 Which Of The Following Is Diamagnetic 1 N2 2 N22 3
Which Of The Following Is Diamagnetic 1 N2 2 N22 3
Molecular Orbital Diagram Wikipedia
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 What Are The Molecular Orbital Configurations For N 2 N 2
What Are The Molecular Orbital Configurations For N 2 N 2
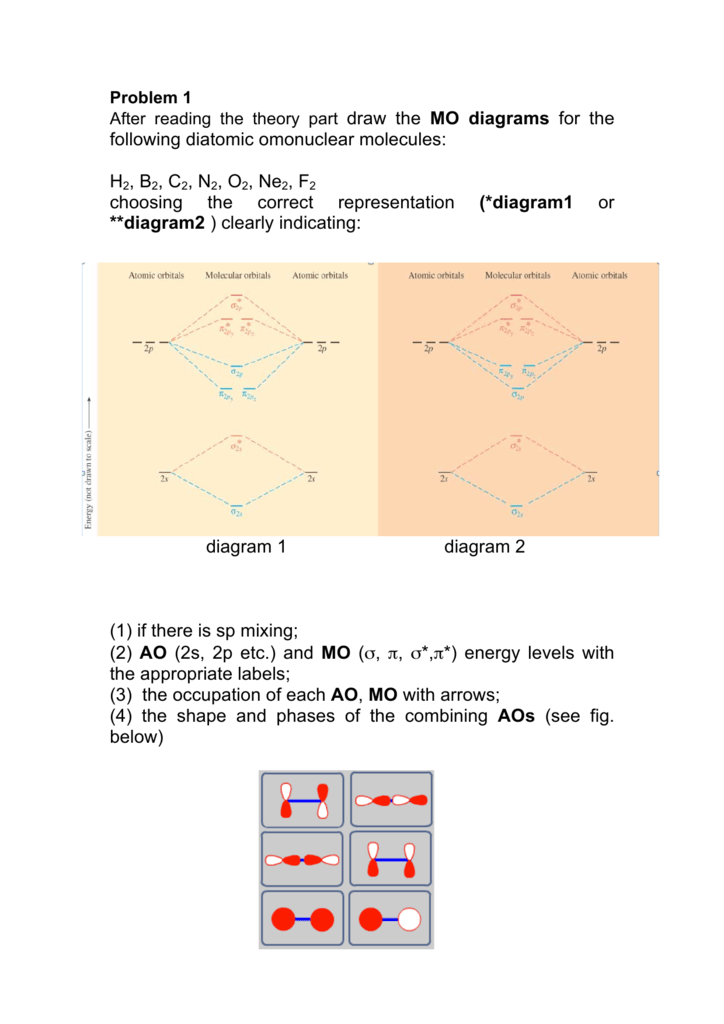 Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
 Molecular Orbitals In Nitrogen Chemtube3d
Molecular Orbitals In Nitrogen Chemtube3d

Belum ada Komentar untuk "N2 2 Molecular Orbital Diagram"
Posting Komentar