Electron Dot Diagram For Magnesium
The nex six electrons will go in the 2p orbital. The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons.
Magnesium phosphide is found on list a which contains most pesticides that are used on foods and hence have a high potential for human exposure.
Electron dot diagram for magnesium. Well put six in the 2p orbital and then put the remaining two electrons in the 3s. The electrons are shown as dots on the outside of the atomic symbol. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for.
Since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same group would have the same electron dot diagram. Therefore the magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Magnesium is in group ii so it has two valence electrons.
Magnesiumhydroxide hmgo cid 5162449 structure chemical names physical and chemical properties classification patents literature biological activities. A beryllium atom with two valence electrons would have the electron dot diagram below. List a consists of the 194 chemical cases or 350 individual active ingredients for which epa issued registration standards prior to fifra 88.
What are the valence electrons for magnesium. There are two types of diagrams one is the lewis diagram the other is the electron dot diagram. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the elements symbol.
In other words if every element in group 1a has 1 valence electron then every lewis electron dot diagram would have one single dot. The electrons are shown as dots on the outside of the atomic symbol. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital.
The lewis dot structure for sulfur is an s with 6 dots which stand for its six valence. I show you where magnesium is on the periodic table and how to determine how many valence electrons magnesium has. The p orbital can hold up to six electrons.
Magnesium is in group ii so it has two valence electrons. A step by step explanation of how to draw the lewis dot structure for mg magnesium.
 Videos Matching Lewis Dot Structure For Beryllium Be Revolvy
Videos Matching Lewis Dot Structure For Beryllium Be Revolvy
 Image Result For Magnesium Chloride Electron Configuration
Image Result For Magnesium Chloride Electron Configuration
 How To Draw The Lewis Dot Structure For Mg No3 2 Magnesium Nitrate
How To Draw The Lewis Dot Structure For Mg No3 2 Magnesium Nitrate
 Bromide Dot Diagram Wiring Diagram Document Guide
Bromide Dot Diagram Wiring Diagram Document Guide
 8 E Exercises Chemistry Libretexts
8 E Exercises Chemistry Libretexts
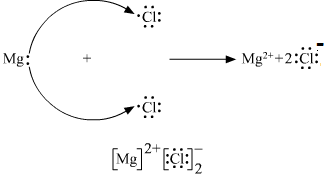 Write The Electron Dot Structure S Of Magnesium And Chlorine
Write The Electron Dot Structure S Of Magnesium And Chlorine
 Write The Electron Dot Structure For Sodium Oxygen And
Write The Electron Dot Structure For Sodium Oxygen And
 I Write The Electron Dot Structures For Sodium Oxygen And
I Write The Electron Dot Structures For Sodium Oxygen And
Lewis Electron Dot Diagrams Magnesium Data Wiring Schemes
 Ionic Bonding Chemistry For Non Majors
Ionic Bonding Chemistry For Non Majors
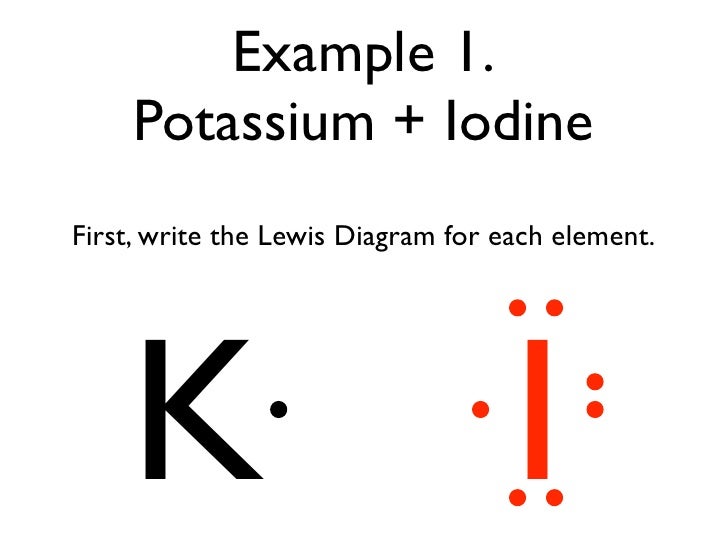
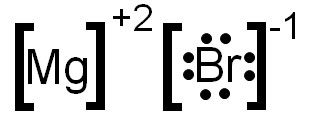 Ionic Bonds Electron Dot Formulas Texas Gateway
Ionic Bonds Electron Dot Formulas Texas Gateway
Magnesium Iodide I2mg Chemspider
Dot Diagram Fluorine Wiring Diagrams
 Electron Dot Diagram For Oxygen Wiring Diagrams Folder
Electron Dot Diagram For Oxygen Wiring Diagrams Folder
Chem4kids Com Magnesium Orbital And Bonding Info
 Lewis Dot Structures Dots Are Arranged Around An Element S
Lewis Dot Structures Dots Are Arranged Around An Element S
Magnesium Fluoride Facts Formula Properties Uses
Electron Dot Diagram Magnesium Shopnext Co
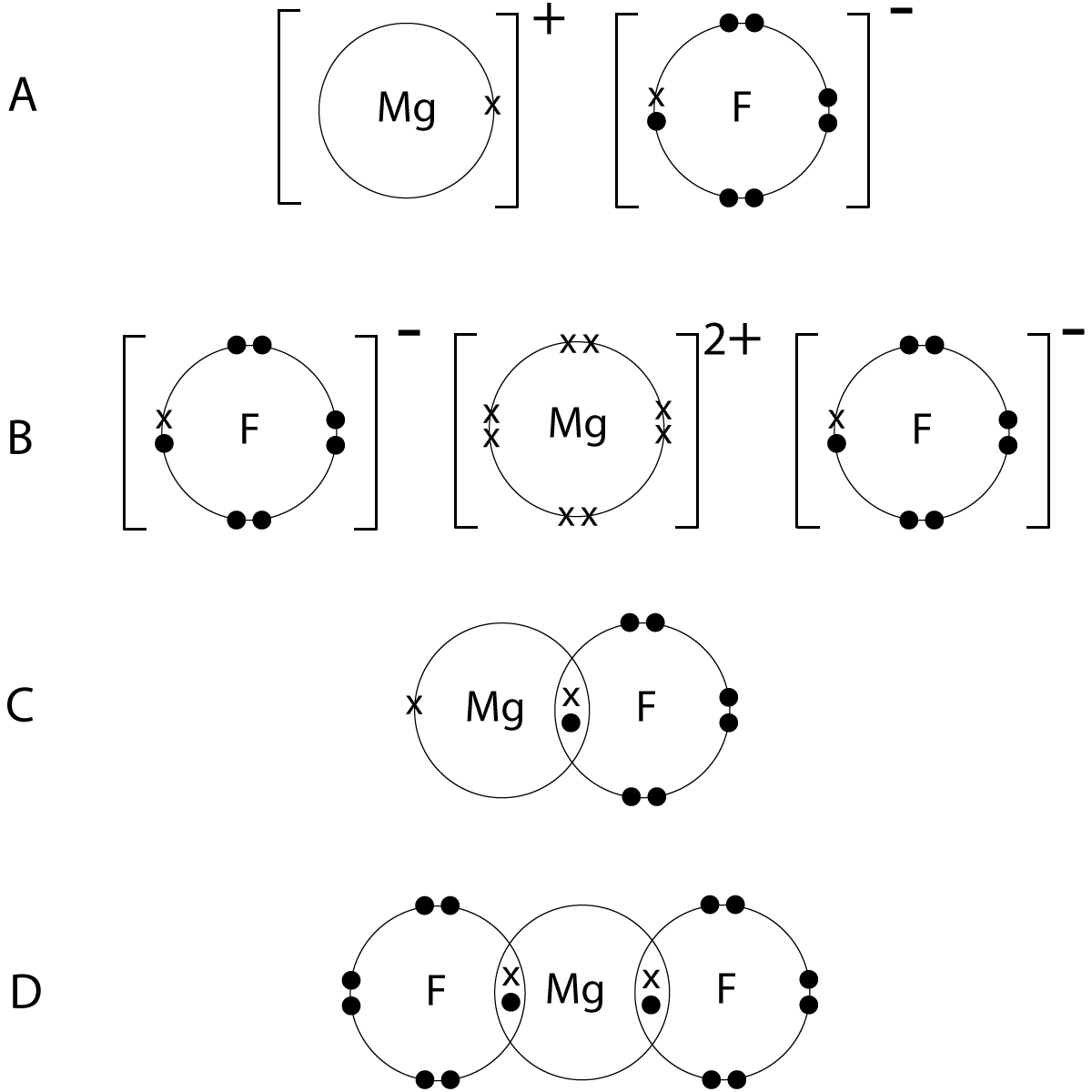
 Dot Cross Diagram Magnesium Fluoride
Dot Cross Diagram Magnesium Fluoride

 Lewis Dot Structure For Magnesium Mg
Lewis Dot Structure For Magnesium Mg
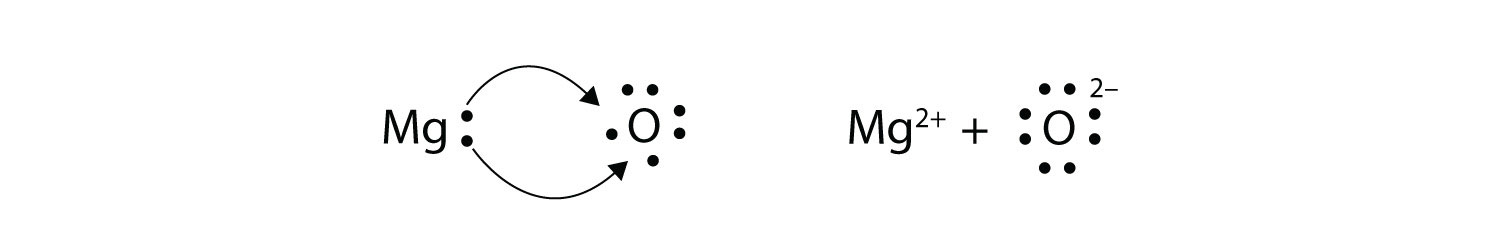

Belum ada Komentar untuk "Electron Dot Diagram For Magnesium"
Posting Komentar