Phase Change Diagram With Equations
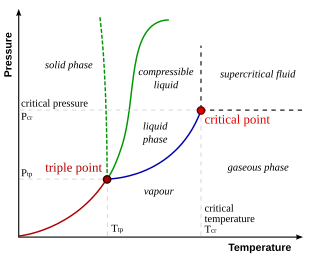
It can also be defined as the transition of water vapour into water droplets upon contacting a solid surface. Point x represents the critical point and at the critical point and beyond the substance is forever in the vapor phase.
Thermodynamics And Thermochemistry Mcat Review
The equilibrium vapor pressure between water vapor to the right of the line and liquid water to the left of the line as calculated by the clausius clapeyron equation.

Phase change diagram with equations. At higher altitudes the air pressure is lower. Heat required to raise the temperature of ice from 10 c to 0 c use the formula q mcδt where q heat energy m mass c specific heat δt change in temperature q 25 gx209 jgc0 c 10 c q 25 gx209 jgcx10 c q 5225 j heat required to raise the temperature of ice from 10 c to 0 c 5225 j step 2. This is an example of a phase diagram.
Condensation is the process by which the physical state of a substance changes from its gas phase to liquid phase. The phase change from vapour or gas to liquid is sometimes particularly in cryogenics named liquefaction. Often when you are asked about a phase transition you will need to refer to a phase diagram to answer it.
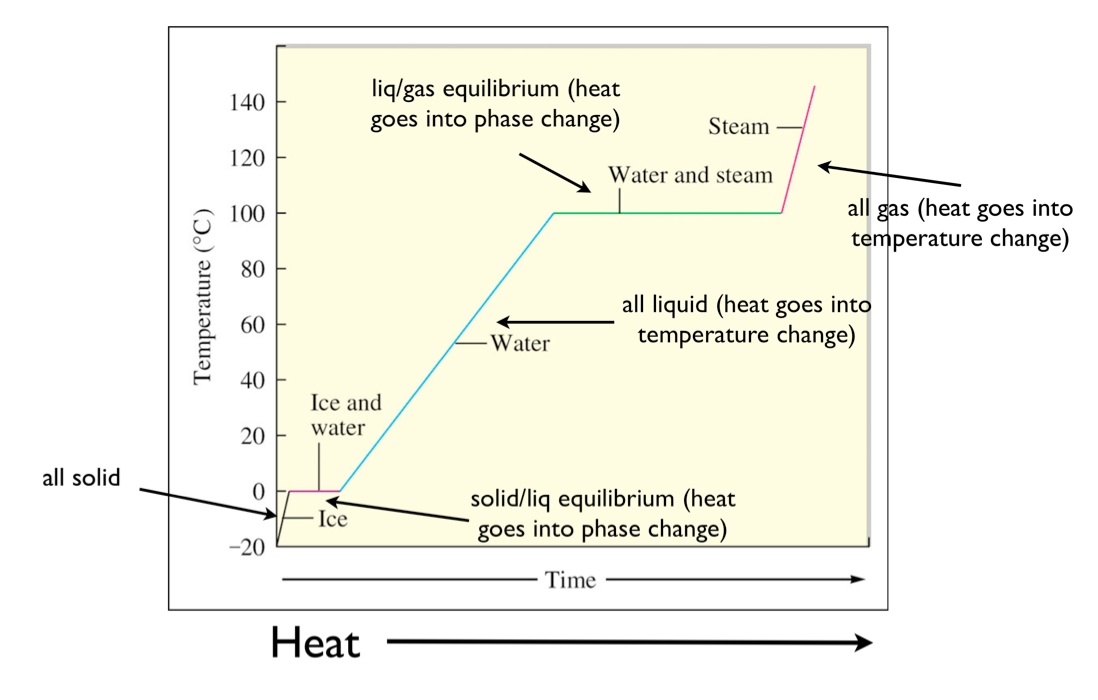
This video contains plenty of examples and practice. This videos explains how to calculate the heat absorbed by a substance as it changes from the solid state to the gaseous state. Phase diagram for water for most water pressures and temperatures that are relevant to the atmosphere.
These diagrams usually have the normal boiling point and normal melting point marked on them and have the pressures on the y axis and temperatures on the x axis. For pure substance in single phase can calculate how much e needed using. Calculating energy changes at phase changes hv and hf it takes energy to heat stuff up.
Q mc t q energy in joules m mass in grams c specific heat capacity t change in temperature tf ti on other hand when something cools down energy is released. This chemistry video tutorial explains the concepts behind the phase diagram of co2 carbon dioxide and the phase diagram of water h2o. The solution is heated making the solvent to evaporate which is then collected separately upon condensation.
Heat required to convert 0 c ice to 0 c water use the formula for heat. Thermodynamics of phase change 5 fig. A phase diagram is a visual representation of how a substance changes phases.
This process is useful in separating a solute and solvent from its solution. Q mδh f where q heat energy m mass δh f heat of. Phase transitions and phase diagrams duration.
This diagram allows us to explain strange phenomena such as why water boils at a lower temperature at higher altitudes for example. Nomenclature for phases and phase changes for a pure substance and p v t diagram.

 Fundamentals Of Phase Transitions Chemistry Libretexts
Fundamentals Of Phase Transitions Chemistry Libretexts
 Solid Diagram List Of Wiring Diagrams
Solid Diagram List Of Wiring Diagrams
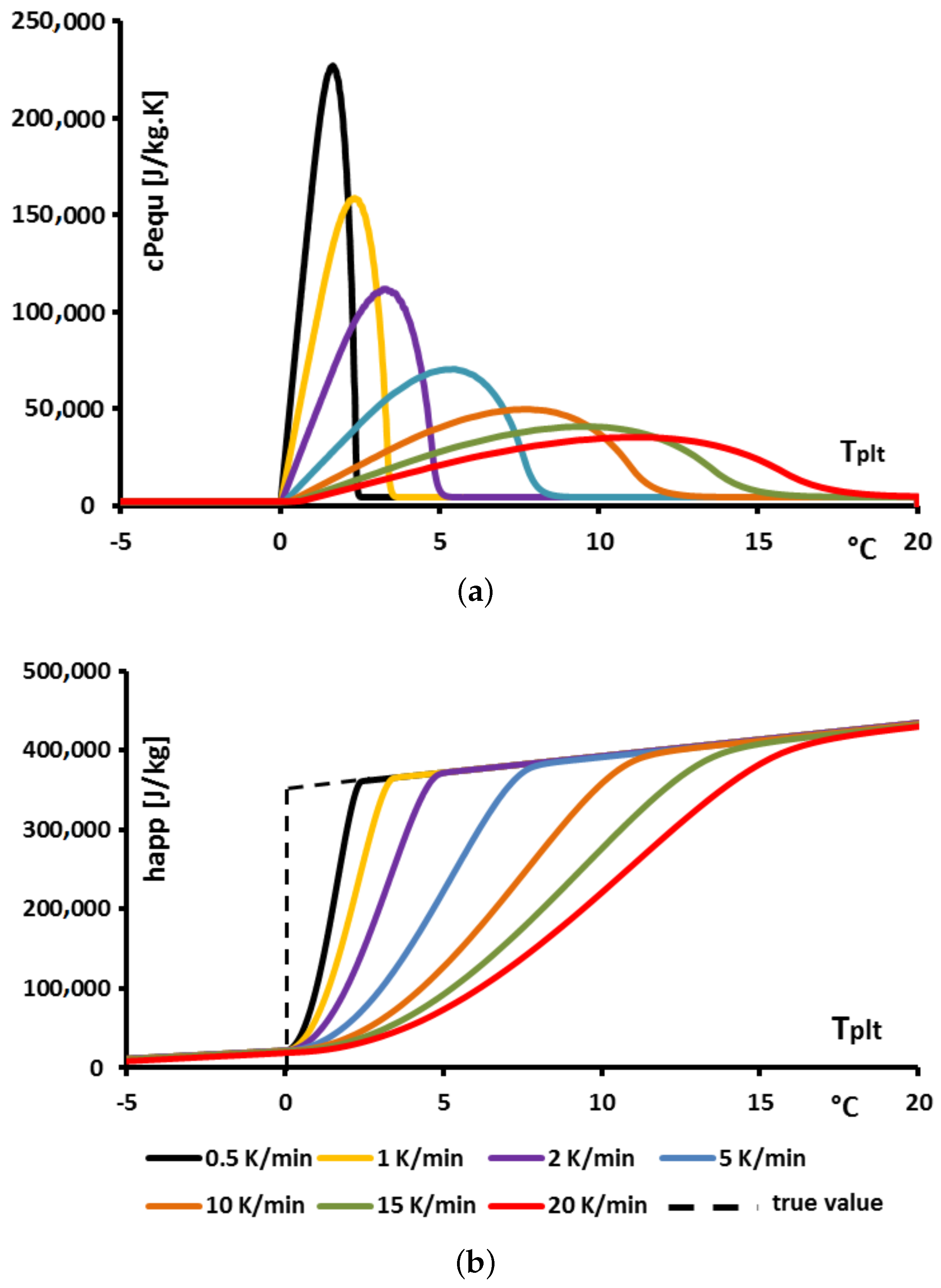 Applied Sciences Free Full Text Challenges Of The Usual
Applied Sciences Free Full Text Challenges Of The Usual
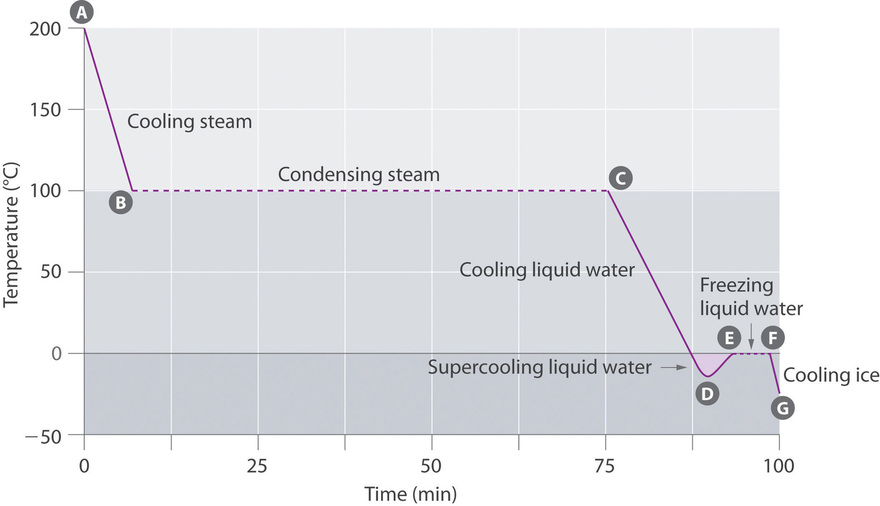 11 4 Phase Changes Chemistry Libretexts
11 4 Phase Changes Chemistry Libretexts
:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png) List Of Phase Changes Between States Of Matter
List Of Phase Changes Between States Of Matter
 Diagram Of Phase Changes International Electrical Diagram
Diagram Of Phase Changes International Electrical Diagram
 Thermomaniac Chapter 3 Property Diagram For Phase Change
Thermomaniac Chapter 3 Property Diagram For Phase Change
 Femtosecond Diffraction Studies Of Solid And Liquid Phase
Femtosecond Diffraction Studies Of Solid And Liquid Phase
 Effect Of Moisture Migration And Phase Change On Effective
Effect Of Moisture Migration And Phase Change On Effective

 Figure 1 13 From 1 Introduction To Nonlinear Differential
Figure 1 13 From 1 Introduction To Nonlinear Differential
 Solved Task 1 The Fundamental Concepts Principles And De
Solved Task 1 The Fundamental Concepts Principles And De
 Quantification Heat And Temperature Can Be Measured And
Quantification Heat And Temperature Can Be Measured And
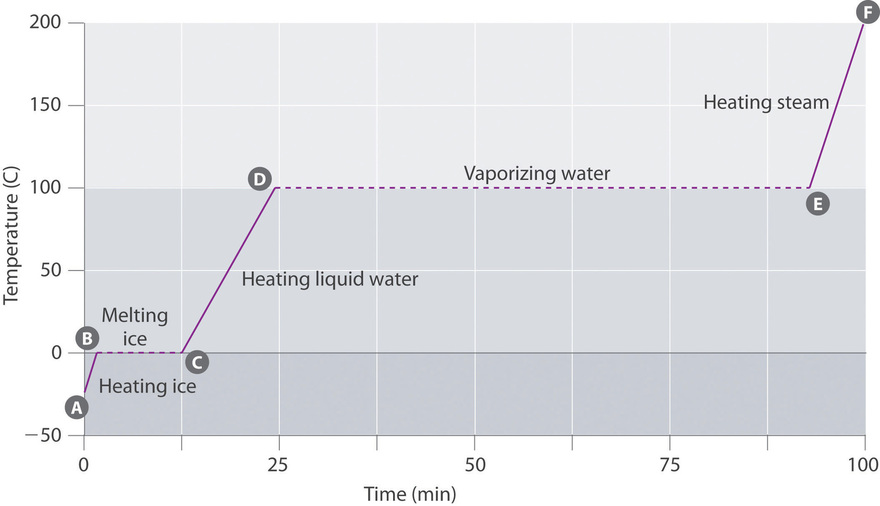 11 4 Phase Changes Chemistry Libretexts
11 4 Phase Changes Chemistry Libretexts
 How Can I Calculate Thermochemistry Equations For Phase
How Can I Calculate Thermochemistry Equations For Phase
 Chapter 2a Pure Substances Phase Change Properties
Chapter 2a Pure Substances Phase Change Properties
What Equations Do We Use For Each Of These Situations
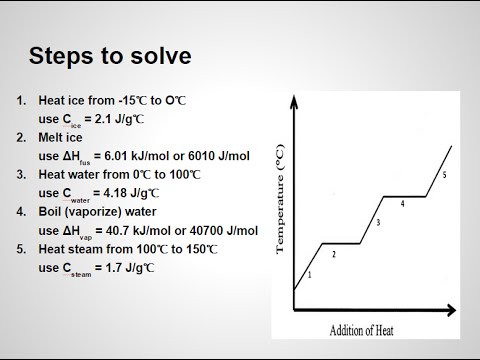 How To Calculate Enthalpy For Phase Changes Of Water Mr Pauller
How To Calculate Enthalpy For Phase Changes Of Water Mr Pauller
Diagram Of Phase Changes Wiring Diagram

Belum ada Komentar untuk "Phase Change Diagram With Equations"
Posting Komentar