Orbital Diagram For Carbon
Draw a molecular orbital diagram for benzene. Since 1s can only hold two electrons the next 2 electrons for c goes in the 2s orbital.
 How Do Yo Write The Orbital Diagram For Oxygen Socratic
How Do Yo Write The Orbital Diagram For Oxygen Socratic
In writing the electron configuration for carbon the first two electrons will go in the 1s orbital.

Orbital diagram for carbon. A sp2 hybridised carbon is called trigonal carbon atom and the hybridisation is known as trigonal hybridisation. Oxygen has four 2 p electrons. A lewis dot structure would have a c.
By hunds rule the electron configuration of carbon which is 1 s 2 2 s 2 2 p 2 is understood to correspond to the orbital diagram shown in c. Describe the structure of benzene in terms of molecular orbital theory. Experimentally it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.
Therefore the c electron configuration will be 1s 2 2s 2 2p 2. State the length of the carbon carbon bonds in benzene and compare this length with those of bonds found in other hydrocarbons. Describe the structure of benzene in terms of resonance.
Unhybridised 2pz orbital is oriented in a plane at right angle to the plane of three hybridised orbitals. The orbital diagram can be derived from the elemental carbons c electron e configuration. Orbital filling diagram for carbon.
Describe the geometry of the benzene molecule. All right lets think about this bond that we formed right here so here we have an overlap of orbitals an overlap of an sp three hybrid orbital form carbon with a un hybridized s orbital from hydrogen here and so this is a head on overlap so were sharing electrons here in this head on overlap. 368 structure of ethene ch2 ch2 both the carbon atoms in ethene are sp2 hybridised.
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. After each 2 p orbital has one electron in it the fourth electron can be placed in the first 2 p orbital with a spin opposite that of the other electron in that orbital. C is configured as a helium he core as he2s2 2p2 2 4.
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. The remaining two electrons will go in the 2p orbital. Exercise 221 draw an orbital diagram for nitrogen z 7.
 Orbital Filling Electron Configurations Where Do These
Orbital Filling Electron Configurations Where Do These
 9 6 Quantum Mechanical Orbitals And Electron Configurations
9 6 Quantum Mechanical Orbitals And Electron Configurations
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Hybridization Uw Madison Department Of Chemistry
 Explain Why The Electron Diagram Orbital Diagram Of Carbon
Explain Why The Electron Diagram Orbital Diagram Of Carbon
 What Is The Orbital Notation For Carbon Study Com
What Is The Orbital Notation For Carbon Study Com
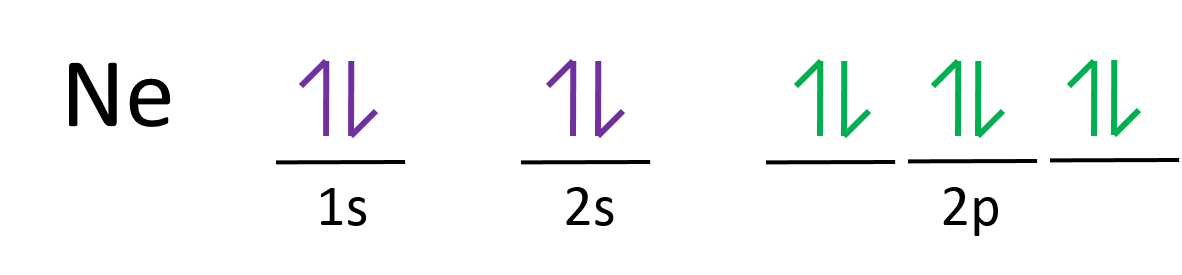 2 2 Electron Configurations Chemistry Libretexts
2 2 Electron Configurations Chemistry Libretexts
 Solved Construct The Orbital Diagram For As Next Click
Solved Construct The Orbital Diagram For As Next Click
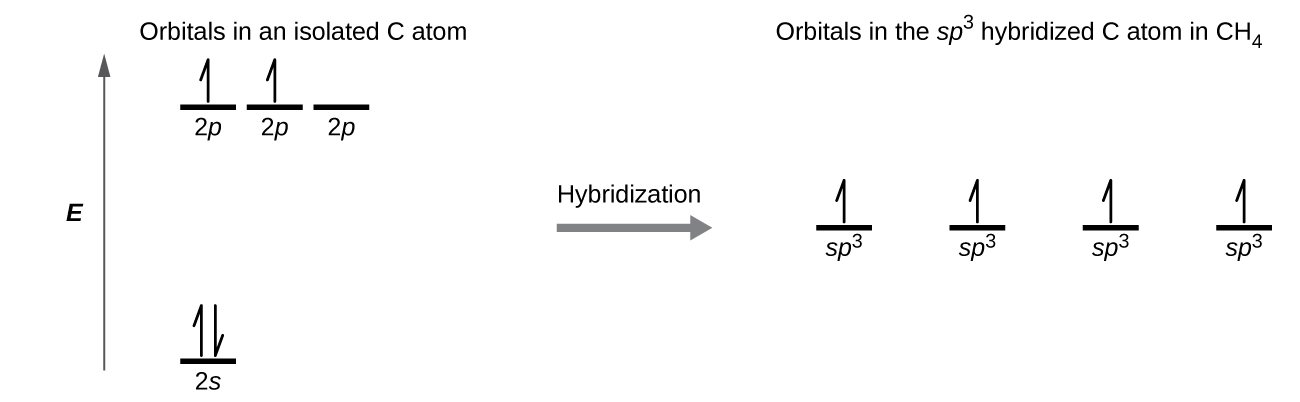 8 2 Hybrid Atomic Orbitals Chemistry
8 2 Hybrid Atomic Orbitals Chemistry
Introduction To Molecular Orbital Theory
 Write Molecular Orbital Configuration Of C2 Predict Magnetic
Write Molecular Orbital Configuration Of C2 Predict Magnetic
 What Is The Electronic Configuration Of Carbon Monoxide In
What Is The Electronic Configuration Of Carbon Monoxide In
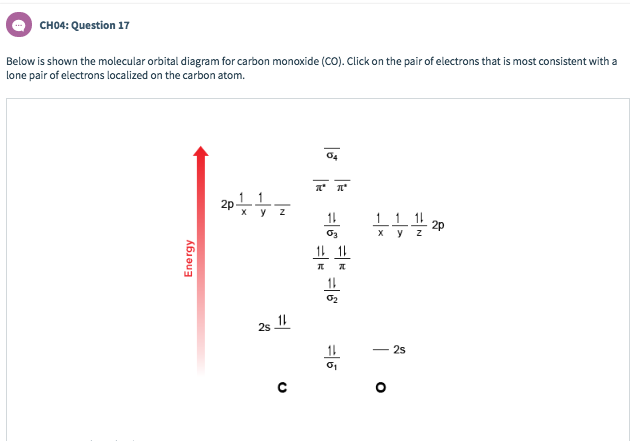 Solved Below Is Shown The Molecular Orbital Diagram For C
Solved Below Is Shown The Molecular Orbital Diagram For C
 Carbon Dioxide Mo Diagram Carbon Dioxide Co2 Molecular O
Carbon Dioxide Mo Diagram Carbon Dioxide Co2 Molecular O
 What Is The Electron Configuration For Carbon What Is The
What Is The Electron Configuration For Carbon What Is The
Molecular Nitrogen And Related Diatomic Molecules
Molecular Structure Atomic Orbitals
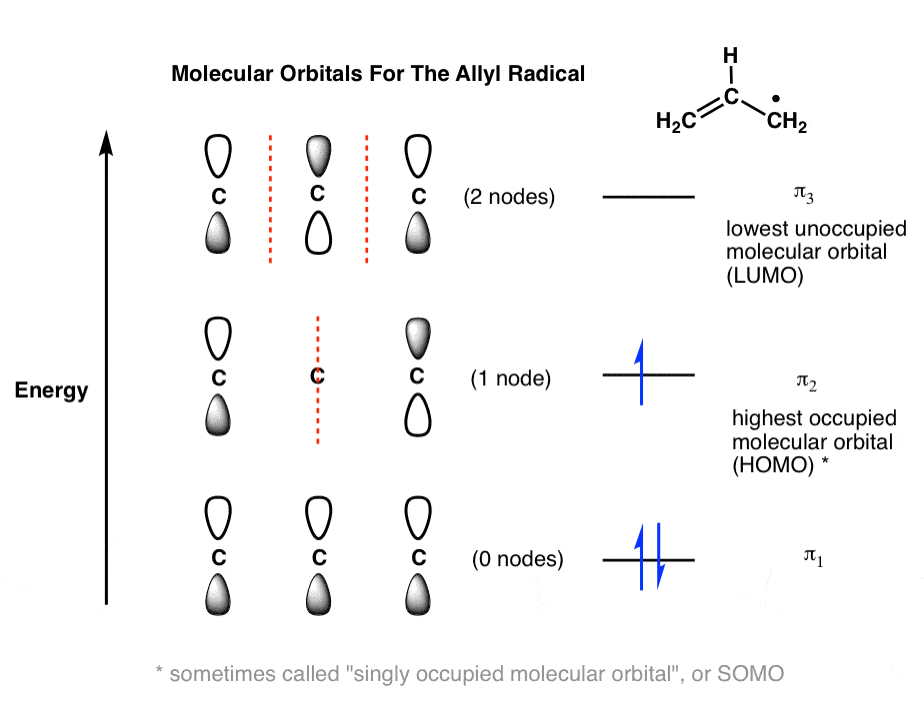 Molecular Orbitals Of The Allyl Cation Allyl Radical And
Molecular Orbitals Of The Allyl Cation Allyl Radical And
 Molecular Orbital A Molecule In Which All The Electrons
Molecular Orbital A Molecule In Which All The Electrons
 What Is The Molecular Orbital Energy Diagram Of Co Quora
What Is The Molecular Orbital Energy Diagram Of Co Quora
 Orbital Diagram Of Carbon Tattoos Teaching Chemistry
Orbital Diagram Of Carbon Tattoos Teaching Chemistry
 Inorganic Chemistry How Can One Tell From The Mo Diagram
Inorganic Chemistry How Can One Tell From The Mo Diagram
 Qualitative Molecular Orbital Diagram For Moc The 10
Qualitative Molecular Orbital Diagram For Moc The 10

Belum ada Komentar untuk "Orbital Diagram For Carbon"
Posting Komentar