Examine The Following Phase Diagram And Determine What Phase Exists At Point D
Vapor which of the following intermolecular forces is the weakest. This preview has intentionally blurred sections.
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
Consider the following phase diagram and identify the process occurring as one goes from point c to point d.
Examine the following phase diagram and determine what phase exists at point d. If we change the composition of the liquid or the temperature the number of phases will be reduced to 2. B the triple point for bo is at a higher temperature than the melting point for bo. Consider the following phase diagram and identify the process occurring as one goes from point c to point d.
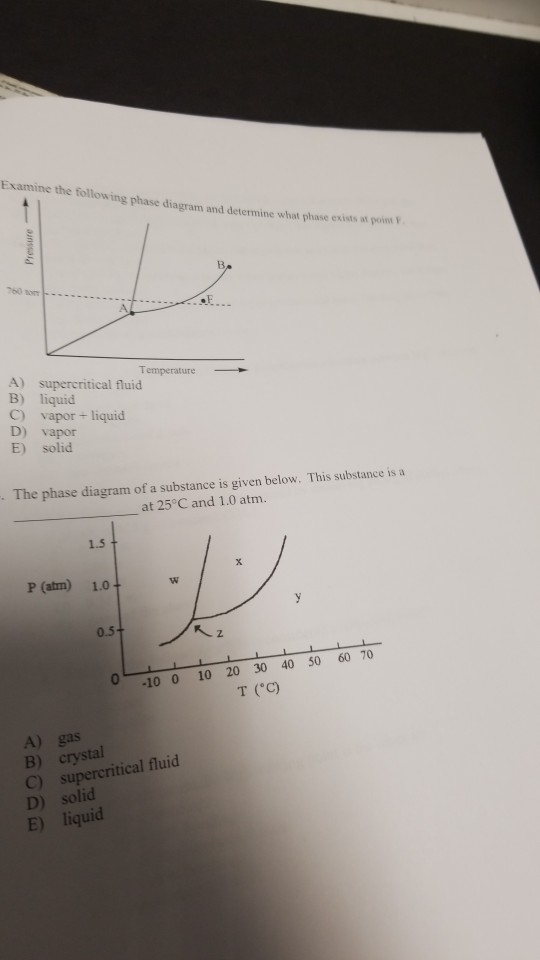
Aincreasing temperature with a phase change from solid to liquid bincreasing temperature with a phase change from solid to vapor cincreasing temperature with a phase change from liquid to vapor dincreasing temperature with no phase change eincreasing temperature beyond the critical point7. See question 10 image a bos has a lower density than bol. Examine the following phase diagram and determine what phase exists at point 760 som temperature a supercritical fluid b liquid c vaporliquid d vapor e solid the phase diagram of a substance i s given below.
Examine the phase diagram for the substance bogusium bo and select the correct statement. B the triple point for bo is at a higher temperature than the melting point for bo. Examine the phase diagram for the substance bogusium bo and select the correct statement.
Increasing temperature with a phase change from solid to vapor examine the following phase diagram and determine what phase exists at point f. C bo changes from a solid to a liquid as one follows the line from c to d. Examine the following phase diagram and identify the feature represented by point a.
The eutectic point is therefore an invariant point. Examine the following phase diagram and identify the feature represented by point a. If the the density of sr metal is 254 gcm3 calculate the number of atoms per unit cell.
Chapter 12 consider the following phase diagram and identify the process occurring as one goes from point c to point d. Gas and liquid 2. Liquids solids and phase changes 15.
A bos has a lower density than bol. Answer to examine the following phase diagram and determine what phase exists at point c. Examine the following phase diagram and identify the.
Sign up to view the full version. Since we looking at a system at constant pressure the phase rule in this case is f c 1 p. C bo changes from a solid to a liquid as one follows the line from c to d.
What type of unit cell is this. Examine the phase diagram for the substance bogusium bo and select the correct statement. Strontium metal crystallizes in a cubic unit cell which has an edge length of 612 pm.
E point b represents the critical temperature and pressure for bo.
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
 A Possible Four Phase Coexistence In A Single Component
A Possible Four Phase Coexistence In A Single Component
 Primitive Phase Diagram For Hydrogen46 Liquid Hydrogen Only
Primitive Phase Diagram For Hydrogen46 Liquid Hydrogen Only
 Solved Examine The Following Phase Diagram And Determine
Solved Examine The Following Phase Diagram And Determine
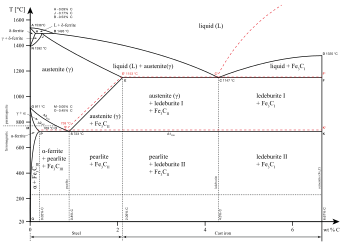
 Delta Iron An Overview Sciencedirect Topics
Delta Iron An Overview Sciencedirect Topics
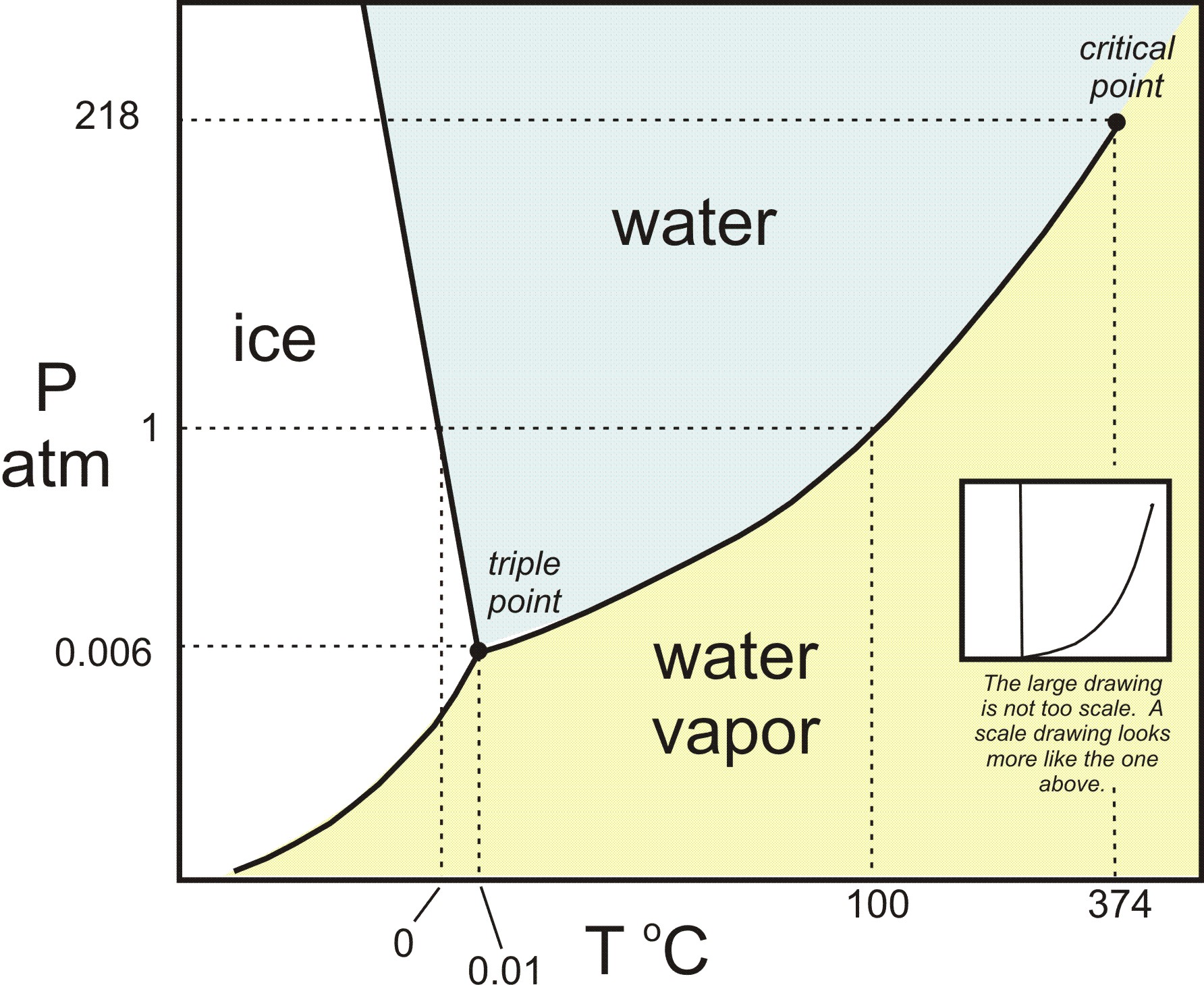
 Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point
Phase Diagrams Of Water Co2 Explained Chemistry Melting Boiling Critical Point
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
/phase-changes-56a12ddd3df78cf772682e07.png) List Of Phase Changes Between States Of Matter
List Of Phase Changes Between States Of Matter
Chem 1412 Su06 Exam 1 Amazon S3 Pages 1 12 Text
Binary Phase Diagrams And Thermodynamic Properties Of
 Assessing Gain And Phase Margins Matlab Simulink
Assessing Gain And Phase Margins Matlab Simulink
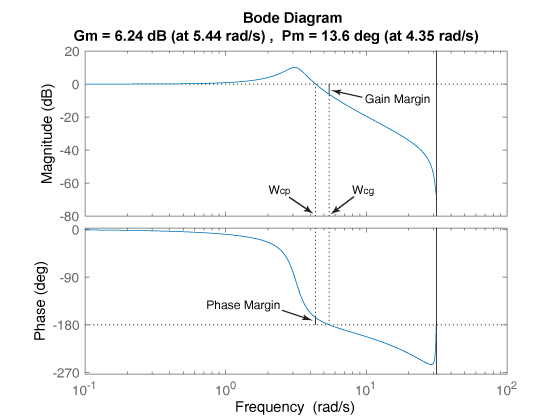 Gain Margin Phase Margin And Crossover Frequencies
Gain Margin Phase Margin And Crossover Frequencies
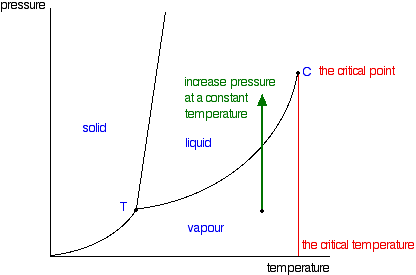 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
Phase Diagrams Video States Of Matter Khan Academy
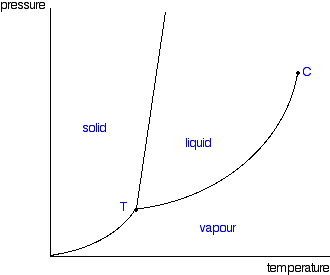 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances


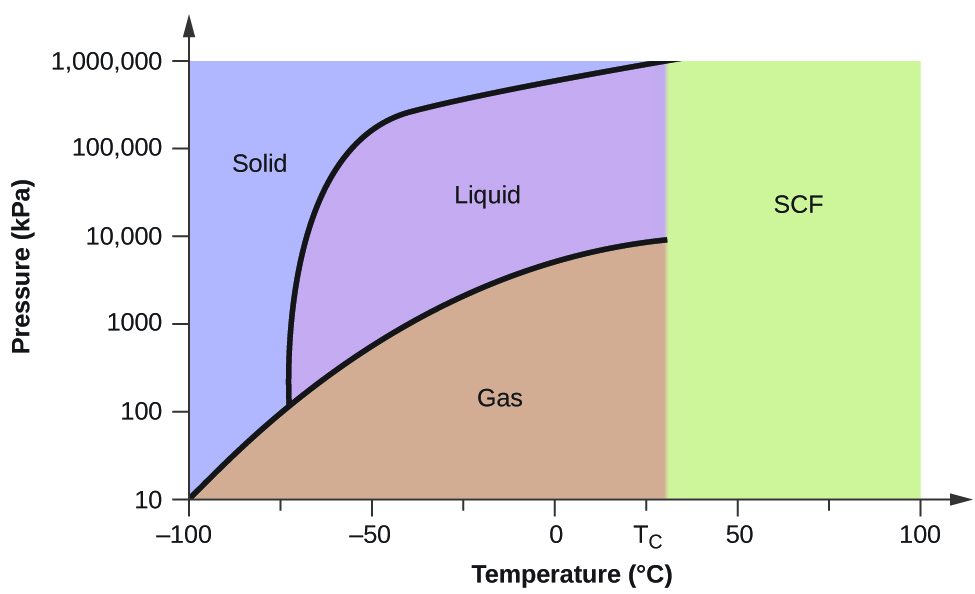




Belum ada Komentar untuk "Examine The Following Phase Diagram And Determine What Phase Exists At Point D"
Posting Komentar