Energy Diagram For Two Step Reaction
The potential energy profiles for three reactions. Please upload a file larger than 100x100 pixels.
 Organic Chemistry 354 Quiz September 25 1998 Dr Sundin
Organic Chemistry 354 Quiz September 25 1998 Dr Sundin
We are experiencing some problems please try again.

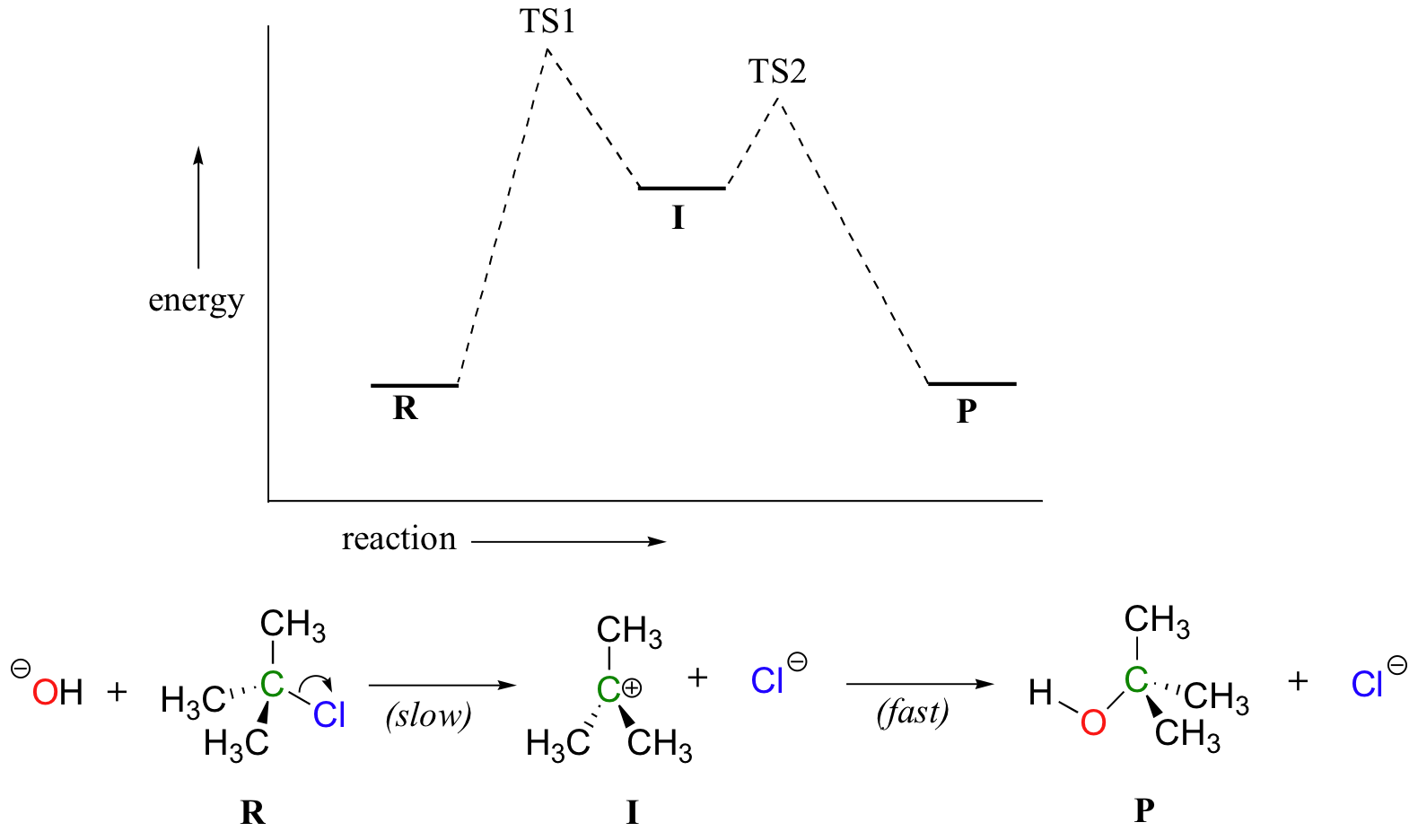
Energy diagram for two step reaction. Because there are two steps involved there are also two transition states and two activation energies to consider as well as the carbocation intermediate. The valley between represents the intermediate for the reaction. Energy diagram for a two step reaction mechanism endothermic because energy is needed to break the a b bond.
Consider the figure below and choose the answer wh. The reaction whose potential energy diagram is shown in the figure is a two step reaction. Positive delta h products at higher energy than starting material in.
Label the energy diagram for a two step reaction. Rate law for sn1 sn2 e1 and e2 reaction potential energy diagram. Energy diagrams of two step reactions.
1 vs 2 step reactions. From the ck 12 foundation christopher auyeung. Indicate dg rxn as well as dg 1 and dg 2 for the first and second activation energies respectively.
Label the energy diagram for a two step reaction. Which of the following statements is true regardin. Now lets turn our attention to a two step reaction mechanism such as our s n 1 reaction between hydroxide and tert butyl chloride.
The energy diagram looks somewhat different. You can only upload files of type png jpg or jpeg. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions.
This is illustrated by the energy diagram where the activation energy for the first step is higher than that for the second step. This problem has been solved. The potential energy diagram shows an activation energy peak for each of the elementary steps of the reaction.
Step one in many reactions more than one step is involved in the formation of products. Draw an energy diagram for a two step reaction that is exothermic overall and consists of a fast but endothermic first step and a slow but exothermic second step. Rates affected by activation energy.
Recall that the first step of the reaction above in which two charged species are formed from a neutral molecule is much the slower of the two steps and is therefore rate determining. Label the energy diagram for a two step reaction. Label the energy diagram for a two step reaction.
It also shows the effect of a catalyst on the forward and reverse activation energy.
 Mechanism Of Reaction And Catalysis Rate And Extent Of
Mechanism Of Reaction And Catalysis Rate And Extent Of
 Label Energy Diagram Schematic Wiring Diagram
Label Energy Diagram Schematic Wiring Diagram
 Exothermic And Endothermic Processes Introduction To Chemistry
Exothermic And Endothermic Processes Introduction To Chemistry
Test1 Ch15 Kinetics Practice Problems

 Mechanisms And Potential Energy Diagrams Chemistry For Non
Mechanisms And Potential Energy Diagrams Chemistry For Non
 Label The Energy Diagram For A Two Step Reaction Beautiful
Label The Energy Diagram For A Two Step Reaction Beautiful
Reaction Mechanisms Grade12uchemistry
 Reaction Mechanism Britannica Com
Reaction Mechanism Britannica Com
 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
 Label The Energy Diagram For A Two Step Reaction Home Work
Label The Energy Diagram For A Two Step Reaction Home Work
Energy Profile Diagram For Two Step Reaction New 50 Fresh
 What Happens To A Molecule While It Is Reacting Chemistry
What Happens To A Molecule While It Is Reacting Chemistry
 Which Of The Following Potential Energy Pe Diagrams Repres
Which Of The Following Potential Energy Pe Diagrams Repres
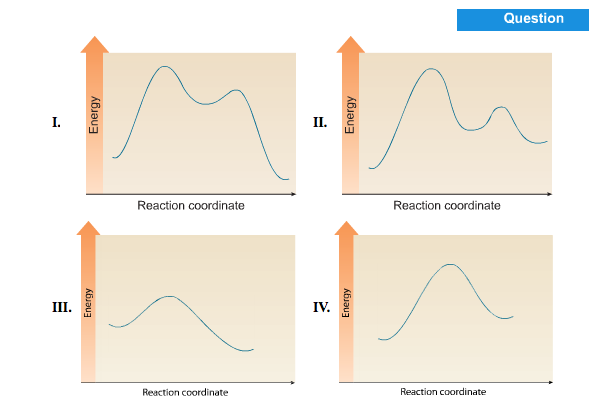 Solved Choose The Energy Diagram For A Two Step Reaction
Solved Choose The Energy Diagram For A Two Step Reaction
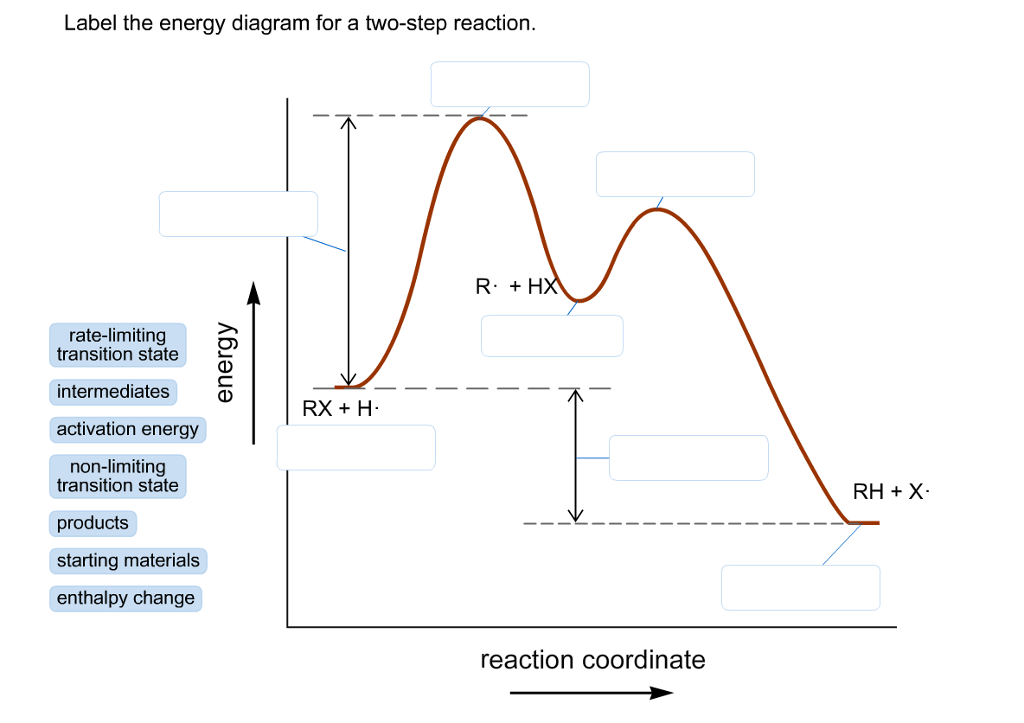 Solved Label The Energy Diagram For A Two Step Reaction
Solved Label The Energy Diagram For A Two Step Reaction
 What Is The Activation Energy For A Reverse Reaction Quora
What Is The Activation Energy For A Reverse Reaction Quora
 2 Reaction Energy Profiles For One Two And Three Step
2 Reaction Energy Profiles For One Two And Three Step
 Figure 3 From Two Step Binding Of O2 To A Vanadium Iii
Figure 3 From Two Step Binding Of O2 To A Vanadium Iii
 Labeling Parts Of A Reaction Coordinate Diagram
Labeling Parts Of A Reaction Coordinate Diagram
Energy Diagram Module Series Part Three Intermediates And
 Francisco Zaera Surface Group Research
Francisco Zaera Surface Group Research
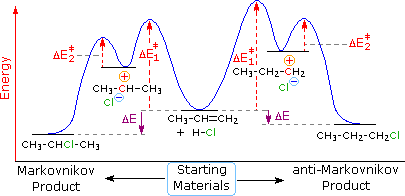

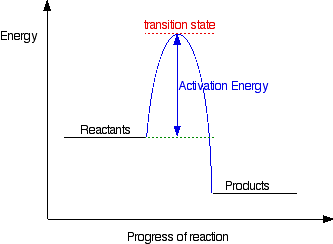
Belum ada Komentar untuk "Energy Diagram For Two Step Reaction"
Posting Komentar