Molecular Orbital Diagram For He2
Fill the molecular orbitals in the energy level diagram beginning with the orbital with the lowest energy. Molecular orbitals of h 2.
 Molecular Orbital Theory Predicting The Stability Of A
Molecular Orbital Theory Predicting The Stability Of A
Ionization potential curves plotted on the diagrams for the neutral systems are nearly parallel to the corresponding orbital energy curves.
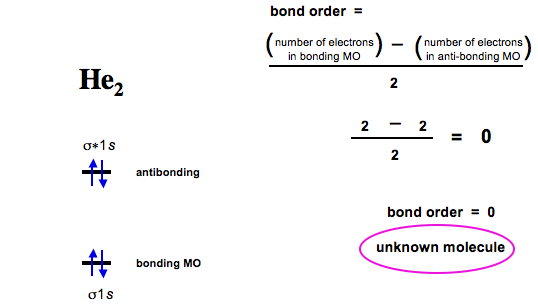
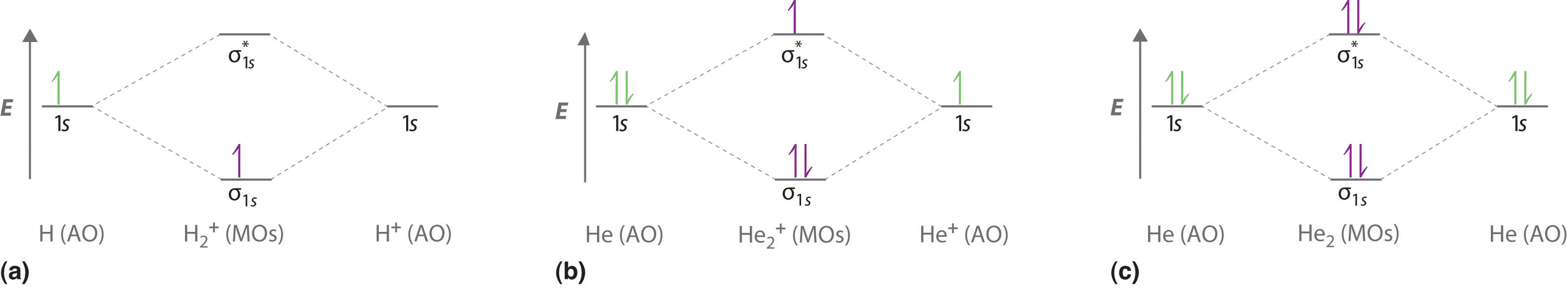
Molecular orbital diagram for he2. Fill in the two orbitals following the aufbau principle. Therefore you have two mos one is the lower energy sigma orbital and the other is the higher energy sigma star antibonding orbital. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b and the central two rung ladder shows the energies of the bonding and antibonding combinations.
Molecular orbital theory bonding antibonding mo bond order homonuclear diatomic molecules duration. Click within the blue boxes to add electrons. 1count the valence electrons that you have for example 3 electrons in he2.
Determine the total number of valence electrons in the he 2 2 ion. The organic chemistry tutor 266209 views. Use an mo diagram to find the bond order and predi.
The molecular orbital approach is one explanation for the ceh h bond. Draw the lewis structure of pf 3a how many share. The molecular orbital energy level diagram which is a diagram that shows the relative energies of molecular orbitals for the h 2 molecule is shown in figure 13.
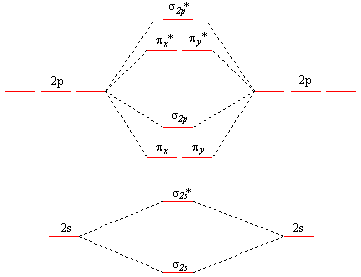
When the 1s wave functions of the two ceh atoms are linearly combined we get a sigma s bonding orbital denoted as s 1s in the diagram herethis approach is called linear combination of atomic orbitals lcao. Click within the blue boxes to add electrons. Construct the molecular orbital diagram for he2 and then identify the bond order.
Construct the molecular orbital diagram for he2. This indicates that koopmans theorem is consistent at all distances and implies that correlation diagrams for neutral molecules should be reasonable approximations to the ionization potential curves. This explanation is based on a mathematical model hence it is a theory.
Combine the two he valence atomic orbitals to produce bonding and antibonding molecular orbitals. Draw the molecular orbital energy level diagram for the system. Molecular orbital energy level diagram bond order and stability.
 Audio 03948795249 Bond Order Bonding Electrons Antibonding
Audio 03948795249 Bond Order Bonding Electrons Antibonding
 What Is The Bond Order Of He2 A 0b C 1d 1 E 2
What Is The Bond Order Of He2 A 0b C 1d 1 E 2
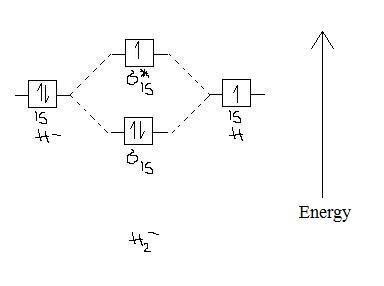 2 3b Mo Theory Of Bonding In H Chemistry Libretexts
2 3b Mo Theory Of Bonding In H Chemistry Libretexts
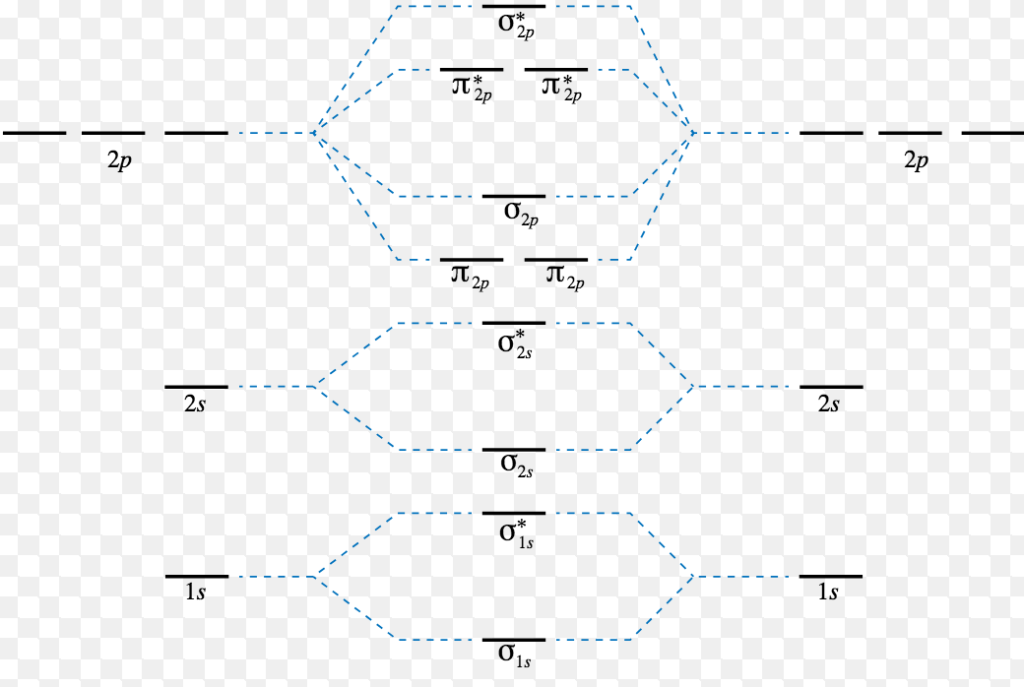 Energy Level Diagram For Molecular Orbitals Chemical
Energy Level Diagram For Molecular Orbitals Chemical
Kinetic And Potential Energy Partitioning For Antibonding

 Molecular Orbital Theory Ii Mo S Of The H2 Molecule
Molecular Orbital Theory Ii Mo S Of The H2 Molecule
Hybridization And Molecular Orbital Mo Theory Pages 1 29
 Use The Molecular Orbital Diagram Shown To Clutch Prep
Use The Molecular Orbital Diagram Shown To Clutch Prep
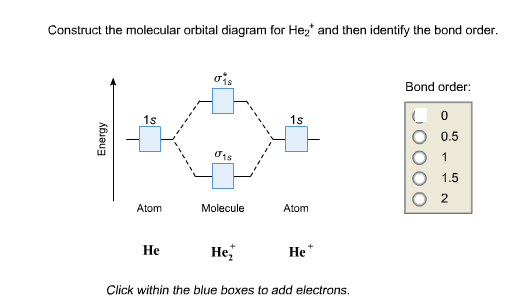 Solved Construct The Molecular Orbital Diagram Fro He 2
Solved Construct The Molecular Orbital Diagram Fro He 2
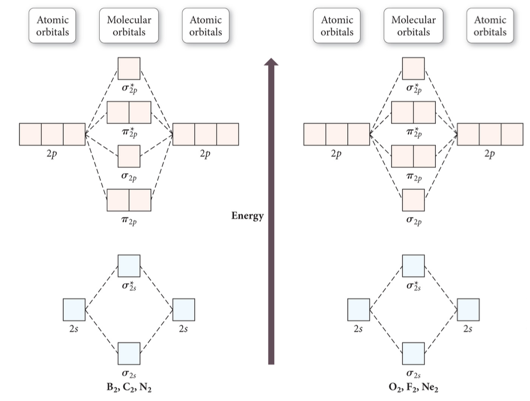
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
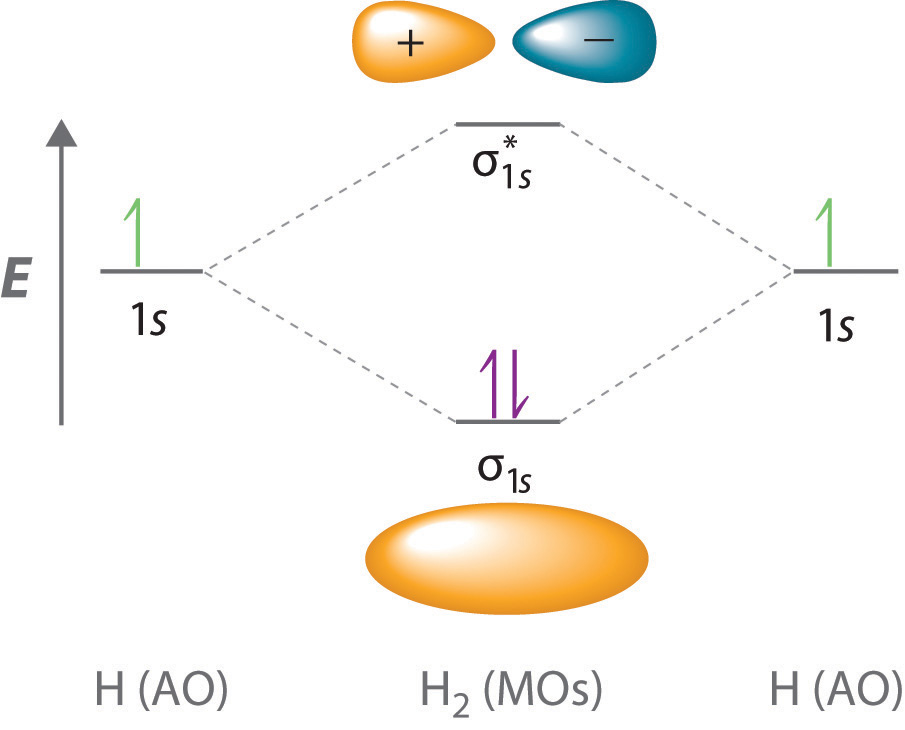 Delocalized Bonding And Molecular Orbitals
Delocalized Bonding And Molecular Orbitals
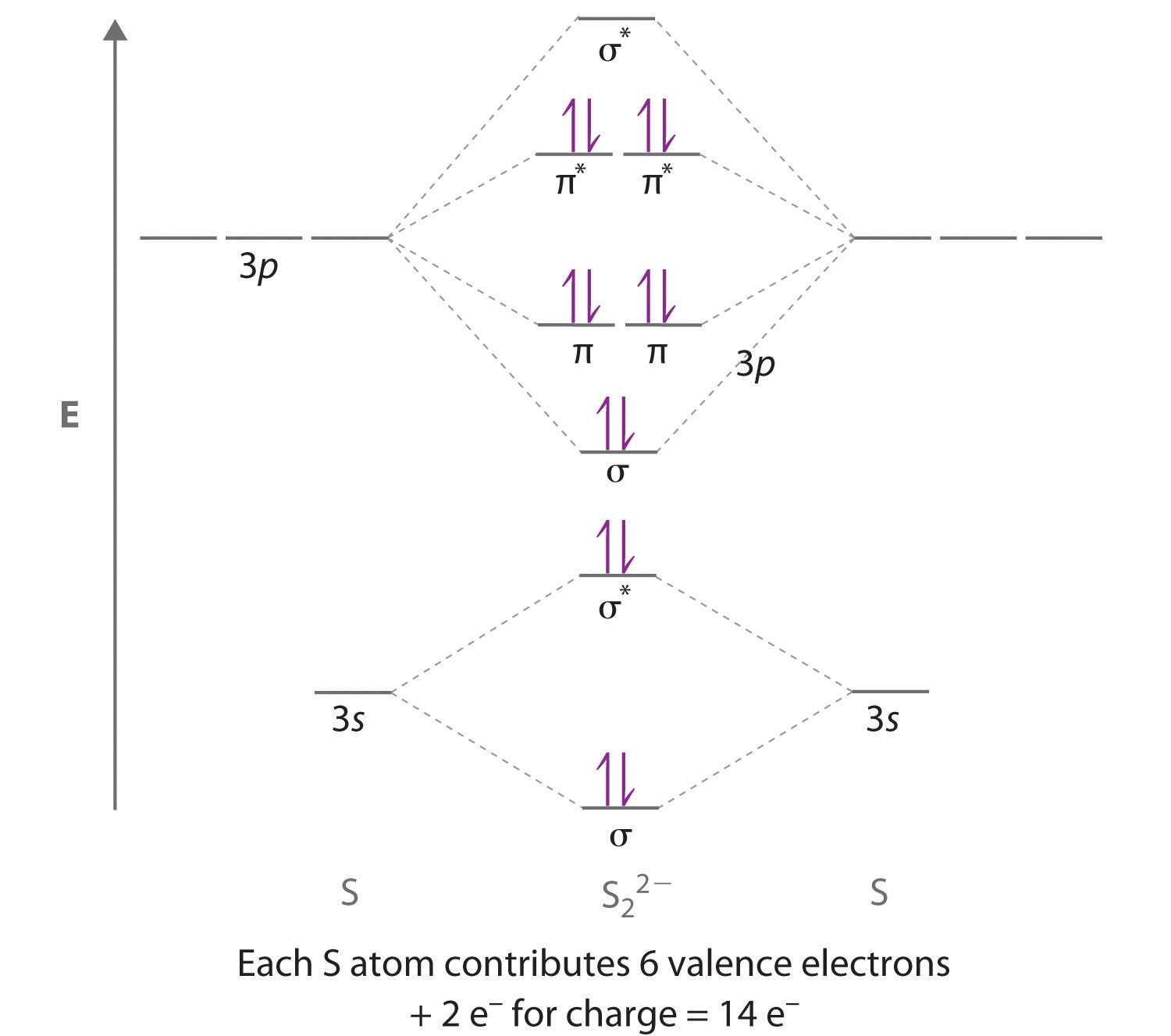 Delocalized Bonding And Molecular Orbitals
Delocalized Bonding And Molecular Orbitals
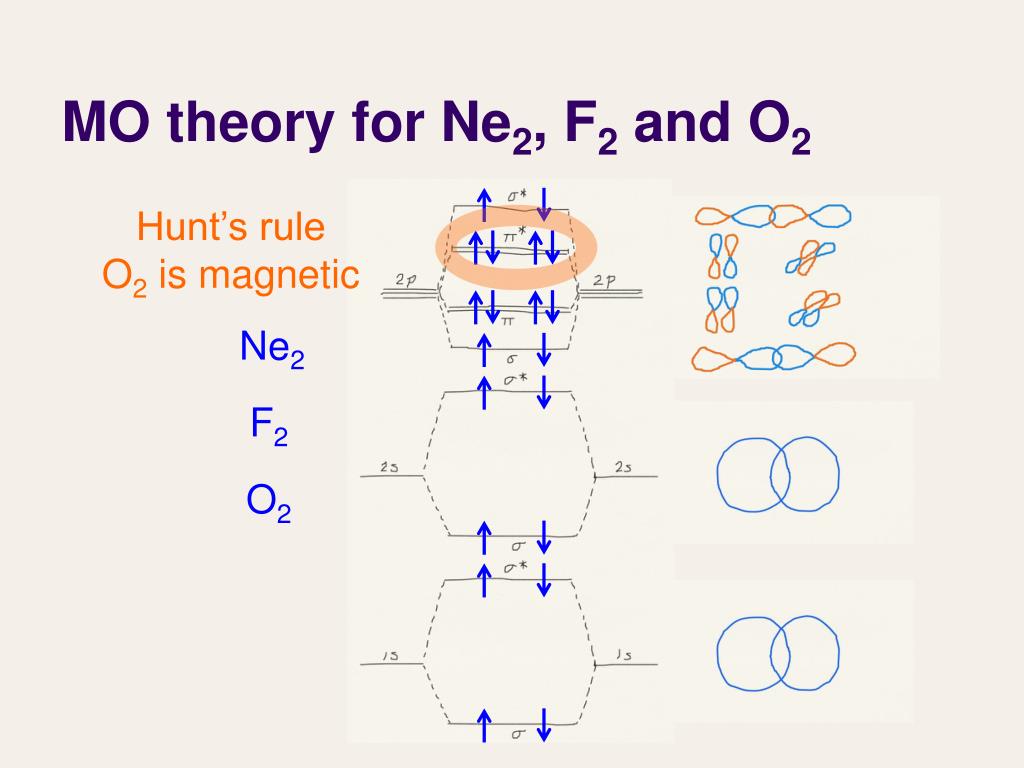 Ppt Lecture 27 Molecular Orbital Theory Iii Powerpoint
Ppt Lecture 27 Molecular Orbital Theory Iii Powerpoint
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
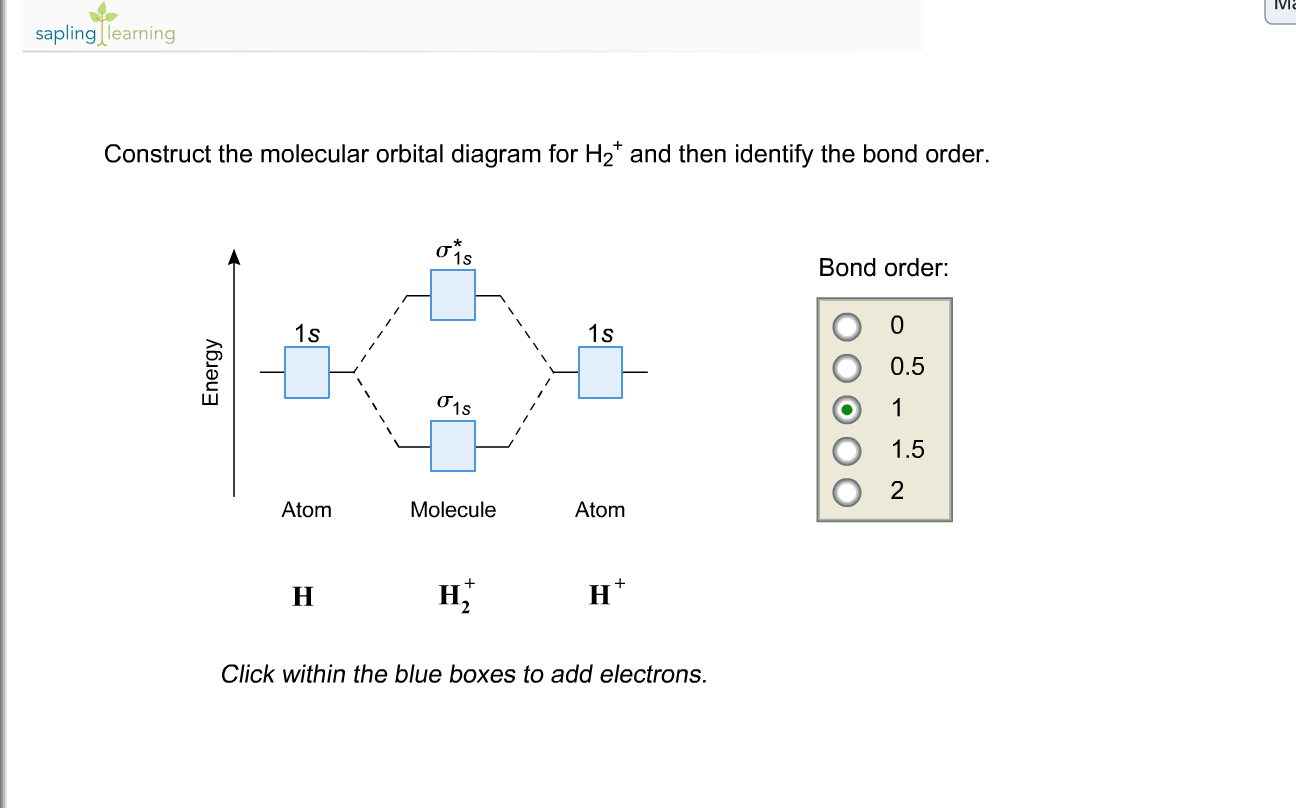 Solved Construct The Molecular Orbital Diagram For H2 An
Solved Construct The Molecular Orbital Diagram For H2 An
 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
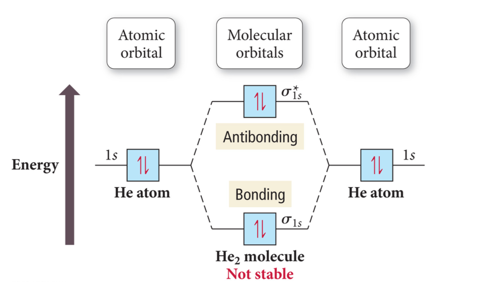
 He2 Explains Why It Doesnt Exist Third Principle Of
He2 Explains Why It Doesnt Exist Third Principle Of
 Bond Order Introduction To Chemistry
Bond Order Introduction To Chemistry
 Chemical Bonding Molecular Orbitals Of H2 And He2
Chemical Bonding Molecular Orbitals Of H2 And He2
According To Molecular Orbital Theory Which Of The


Belum ada Komentar untuk "Molecular Orbital Diagram For He2"
Posting Komentar