What Is A Lone Pair In A Lewis Diagram
Ideally the three pairs of electrons should arrange themselves trigonally around the sn atom giving an angle of 120 between electron pairs and hence between the two cl atoms. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding.
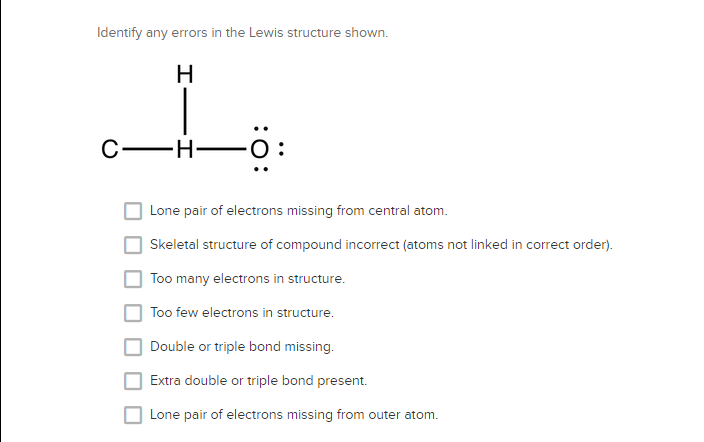 Solved Identify Any Errors In The Lewis Structure Shown C
Solved Identify Any Errors In The Lewis Structure Shown C
And because the non bonding nitrogen lone pair lies fairly close to nitrogen it compresses the h n h bond down from 1095 at to approx.

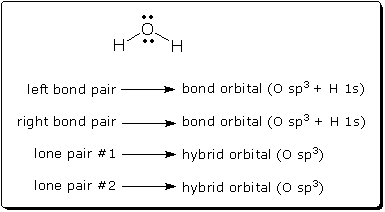
What is a lone pair in a lewis diagram. Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures leds are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Thus the number of lone pair electrons plus the number of bonding electrons equals the total number of valence electrons around an atom. They can be identified by using a lewis structure.
The reason is that the lone pair prefers one of. If we draw the lewis diagram though we find a lone pair as well as two bonding pairs in the valence shell of the sn atom. This electron arrangement is known as trigonal bipyramidal the shape is like a seesaw.
Lines are drawn between atoms that are bonded to one another pairs of dots can be used instead of lines. On other hand the lone pair explains the basicity of the ammonia molecule. Excess electrons that form lone pairs are represented as pairs of dots and are placed next to the atoms.
Organic chemistry lewis structures and. Sf4 lewis structure looks like this. What are lone pairs and how are they represented in a lewis dot diagram.
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. This chemistry video tutorial provides a basic introduction into drawing lewis dot structures but most importantly it provides an explanation on how to calculate the number of lone pairs using a. Lewis diagrams aka lewis structures lewis dot structures lewis dot diagrams are useful because they use simple drawings to show how atoms share valence electrons in molecules polyatomic ions.
The lewis structure of sf4 is the combination of 34 valence electron and 5 electron pairs around the sulfur in which there are four bonding pairs and one lone pair.
 Lewis Structure Brilliant Math Science Wiki
Lewis Structure Brilliant Math Science Wiki
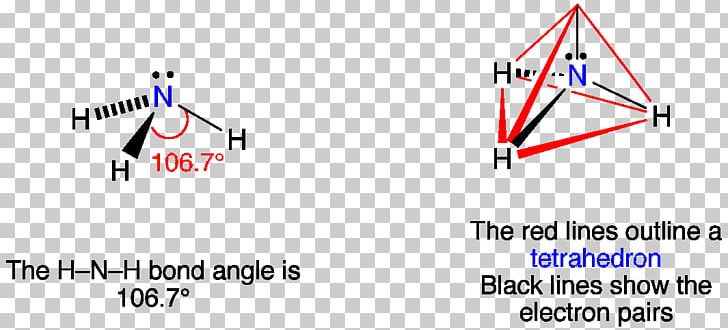 Lewis Structure Vsepr Theory Molecular Geometry Ammonia Lone
Lewis Structure Vsepr Theory Molecular Geometry Ammonia Lone
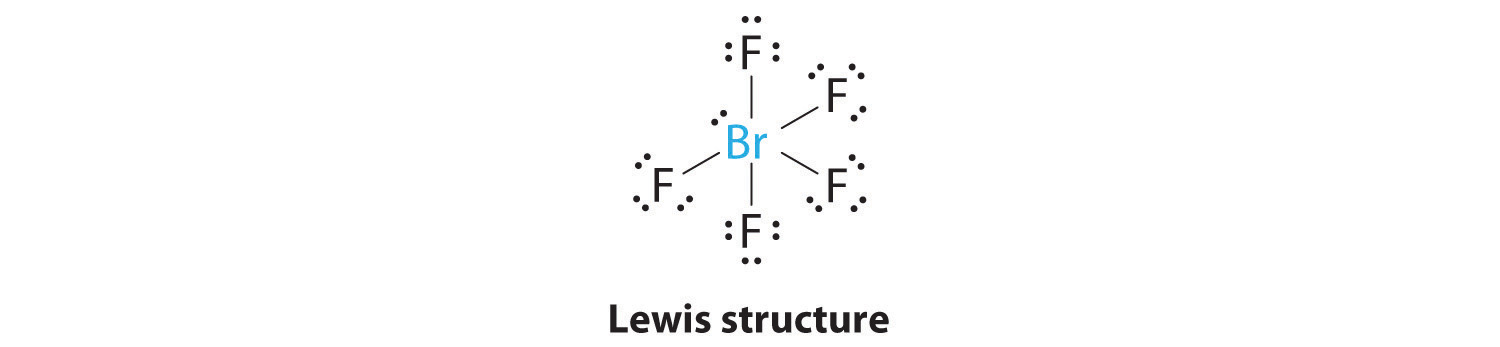 Predicting The Geometry Of Molecules And Polyatomic Ions
Predicting The Geometry Of Molecules And Polyatomic Ions
Illustrated Glossary Of Organic Chemistry Lewis Structure
 How To Draw Lewis Dot Structure
How To Draw Lewis Dot Structure
 Complete A Lewis Structure For S2o32 By Adding Lone Pairs
Complete A Lewis Structure For S2o32 By Adding Lone Pairs
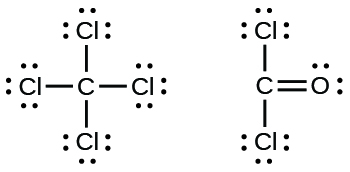 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
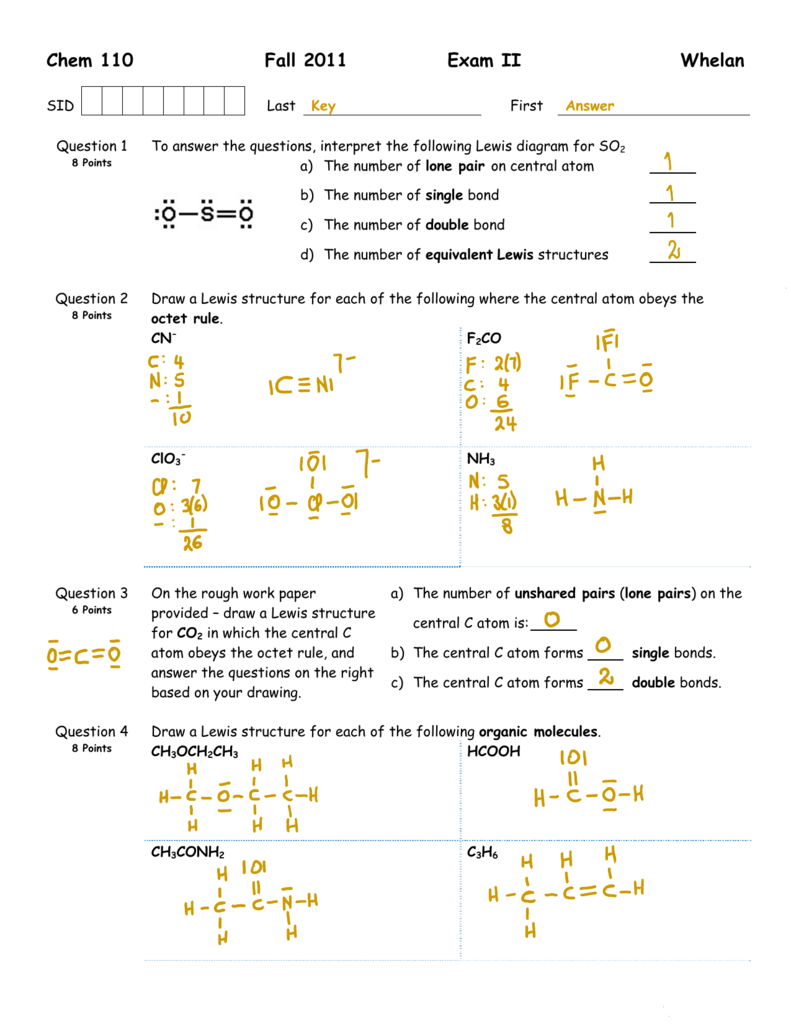
 In The Lewis Structure For Formic Acid Hcooh How Many Bonding Pairs And Lone Pairs Of Electrons Are Present A 4 Bonding 2 Lone B 4 Bonding 5 Lone C 5 Bonding 0 Lone D 5 Bonding 4 Lone
In The Lewis Structure For Formic Acid Hcooh How Many Bonding Pairs And Lone Pairs Of Electrons Are Present A 4 Bonding 2 Lone B 4 Bonding 5 Lone C 5 Bonding 0 Lone D 5 Bonding 4 Lone
 Asf3 Lewis Structure How To Draw The Lewis Structure For Arsenic Trifluoride
Asf3 Lewis Structure How To Draw The Lewis Structure For Arsenic Trifluoride
 Inorganic Chemistry Cyanide Ion Non Bonding Lone Pair
Inorganic Chemistry Cyanide Ion Non Bonding Lone Pair
 Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula
Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula
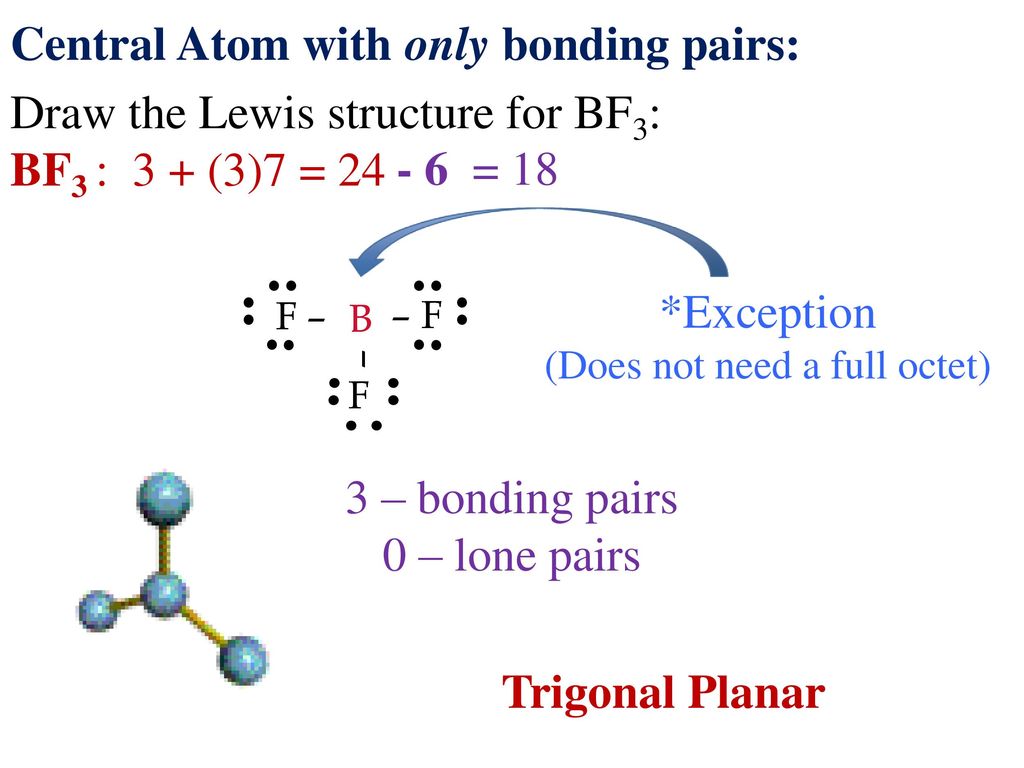 Drawing Lewis Structures And Vsepr Ppt Download
Drawing Lewis Structures And Vsepr Ppt Download
 Lewis Diagram Hcooh Schematics Online
Lewis Diagram Hcooh Schematics Online
Lewis Diagrams For Compound Formation
Lewis Dot Structure For Ccl4 Blog
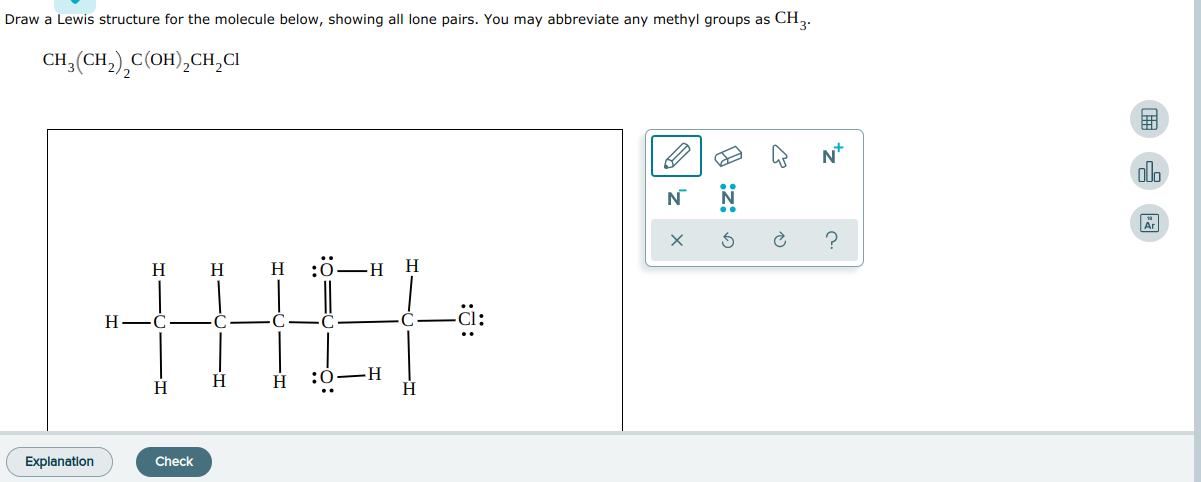 Solved Draw A Lewis Structure For The Molecule Below Sho
Solved Draw A Lewis Structure For The Molecule Below Sho
 5 Steps Lewis Dot Structure How To Do Draw Lewis Dot
5 Steps Lewis Dot Structure How To Do Draw Lewis Dot
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Belum ada Komentar untuk "What Is A Lone Pair In A Lewis Diagram"
Posting Komentar