Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The 3rd Electron Of The N Atom
N 3 l 2 3. How to write electron configurations and orbital diagrams duration.
Following the 2s sublevel is the 2p and pp sublevels always consist of three orbitals.

Use the orbital diagram for nitrogen to write quantum numbers for the 3rd electron of the n atom. L 1 for a p orbital. N 6 to n 4. Orbital filling diagram for carbon.
The first shell n 1 can hold 2 electrons in one orbital labelled 1s. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron. Which sketch represents an orbital with the quantum numbers n 3 l 0 ml 0.
One labelled 2s and three labelled 2p. N 2 l 0 2. The three orbitals of the 3p sublevel will each fill with an up spin electron first the left most orbital will get also down spin electron.
Think about your result. N 1 l 0 4. It also shows you how to find the 4 quantum numbers for an electron and how to write the electron configuration in addition to how to write the orbital notation or fill in the arrows in the.
All three orbitals need to be drawn even if one or more is unoccupied. N 2 l 1 5. This is electron 16 so its third quantum number is 1.
Which one of the following is the correct orbital diagram for ground state nitrogen 7n. Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well as giving each orbital its own line to represent the spins of the electrons too. N 4 l 3.
The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number. An oxygen atom has a total of 8 elections. Match each set of quantum numbers to the correct subshell description by typing in the correct number.
The diagram shows the number of subshell by using boxes or lines for electrons use three for p orbitals five for d orbitals and 7 for f orbitals. How do you write the 4 quantum numbers for each of the 8 electrons in the ground state. Electron configuration 1s 2 2s 2 2p 2.
1 s 2 p 3 d and 4 f for the orbital and the superscript number tells you how many electrons are in that orbital. Which one of the following electron transitions in a hydrogen atom results in the greatest release of energy from the hydrogen atom. Symbol ms specifies the spin of an electron and can have values of ½ or ½.
We use s0 p1 d2 f3 the third quantum number tells you which orbital of the sublevel an electron is in.
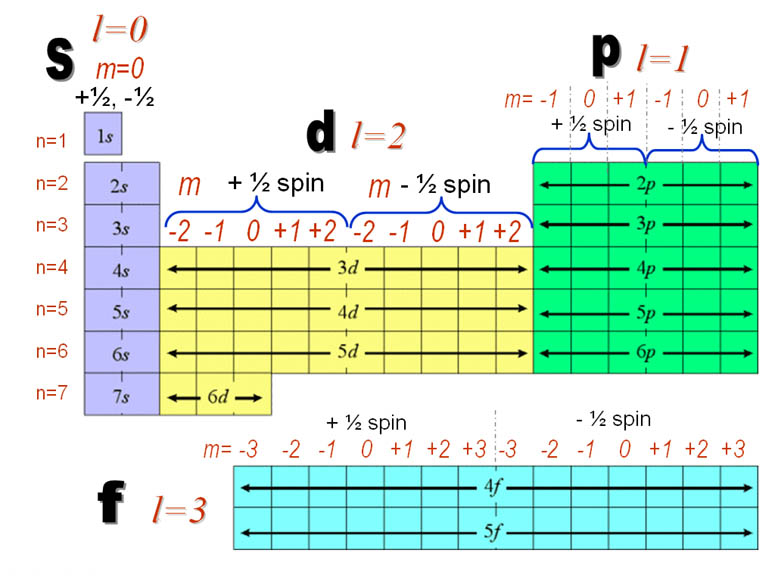 Quantum Numbers Are Just Like Address Of Electron In An Atom
Quantum Numbers Are Just Like Address Of Electron In An Atom


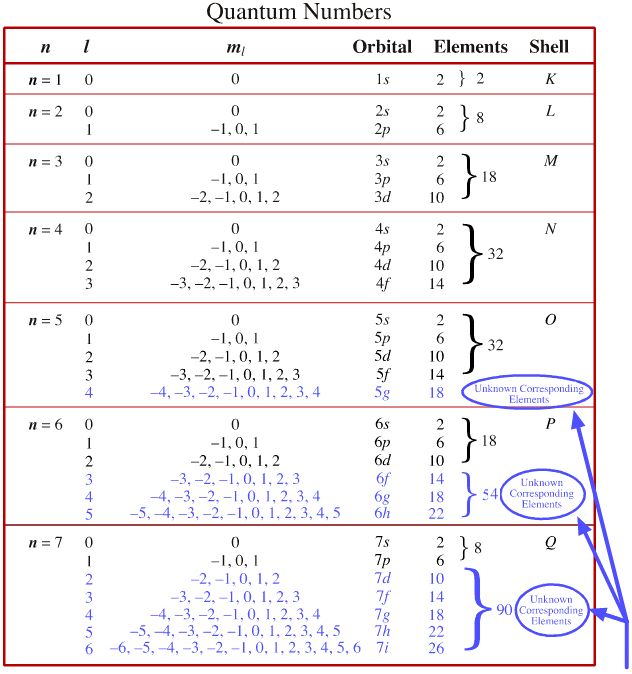 Quantum Number Periodic Table Chemogenesis
Quantum Number Periodic Table Chemogenesis
 Spin Quantum Number Definition Example Video Lesson
Spin Quantum Number Definition Example Video Lesson
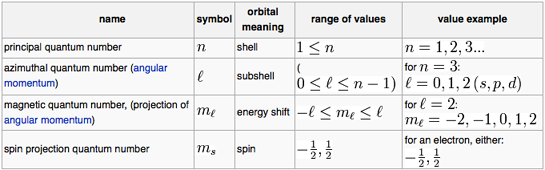 Quantum Number Periodic Table Chemogenesis
Quantum Number Periodic Table Chemogenesis
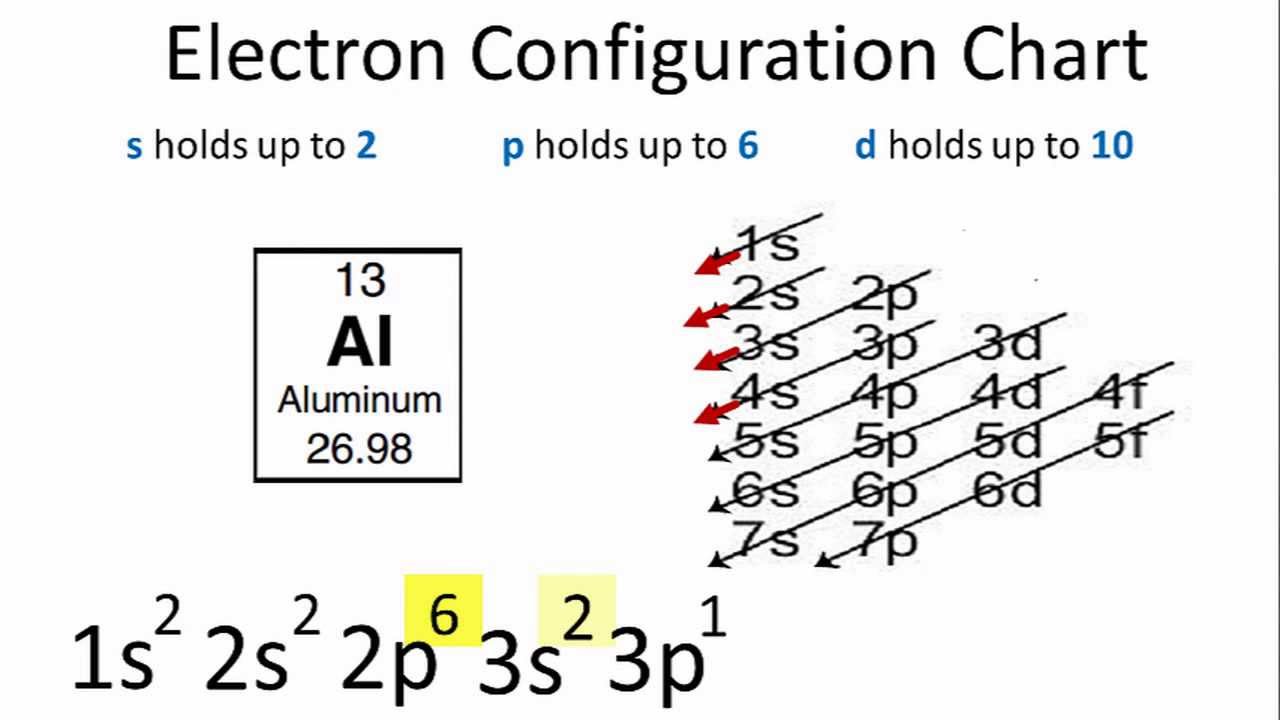 Electron Configuration For Aluminium Al
Electron Configuration For Aluminium Al
 The Pauli Exclusion Principle Physics
The Pauli Exclusion Principle Physics
Pplato Flap Phys 8 3 Multi Electron Atoms
Electron Configurations How To Write Out The S P D F
 Bohr Model Description Development Britannica Com
Bohr Model Description Development Britannica Com
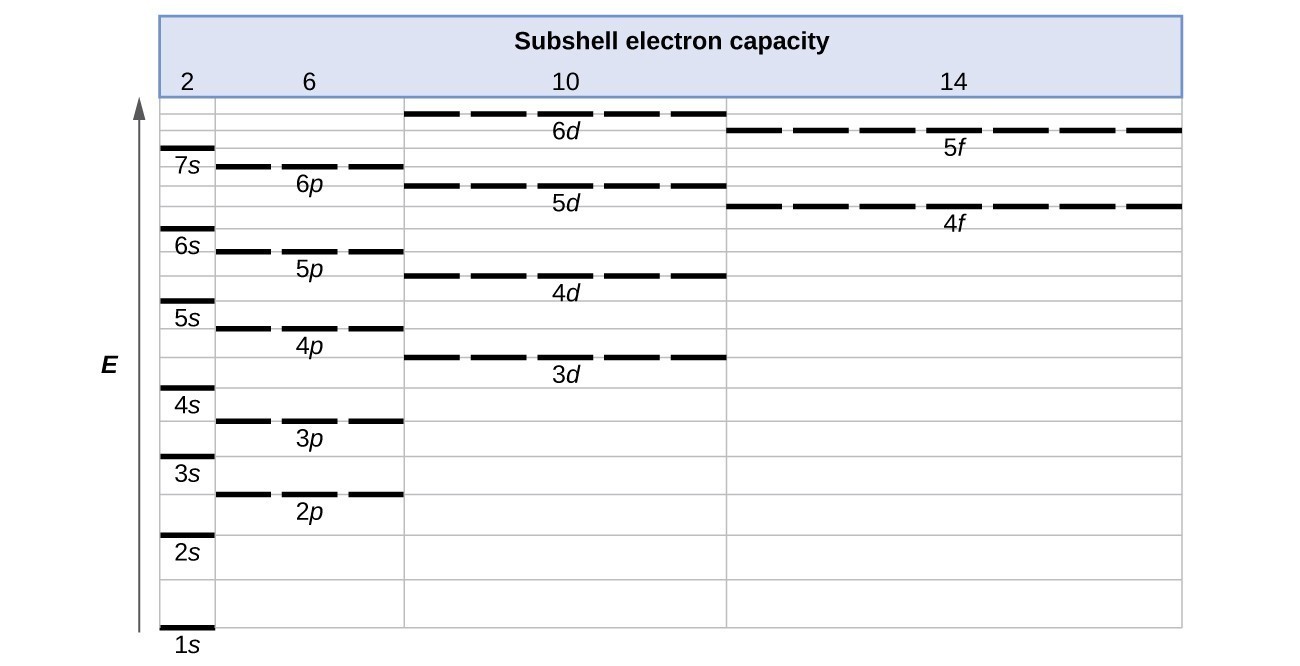 Electronic Structure Of Atoms Electron Configurations
Electronic Structure Of Atoms Electron Configurations
 The Partial Orbital Diagram Below Where N Could Be Any Valid Quantum Number Describes The Valence Electrons For Which Of The Atoms Below A Nitrogen B Silicon C Bromine D Sulfur E
The Partial Orbital Diagram Below Where N Could Be Any Valid Quantum Number Describes The Valence Electrons For Which Of The Atoms Below A Nitrogen B Silicon C Bromine D Sulfur E
 High School Chemistry Orbital Configurations Wikibooks
High School Chemistry Orbital Configurations Wikibooks
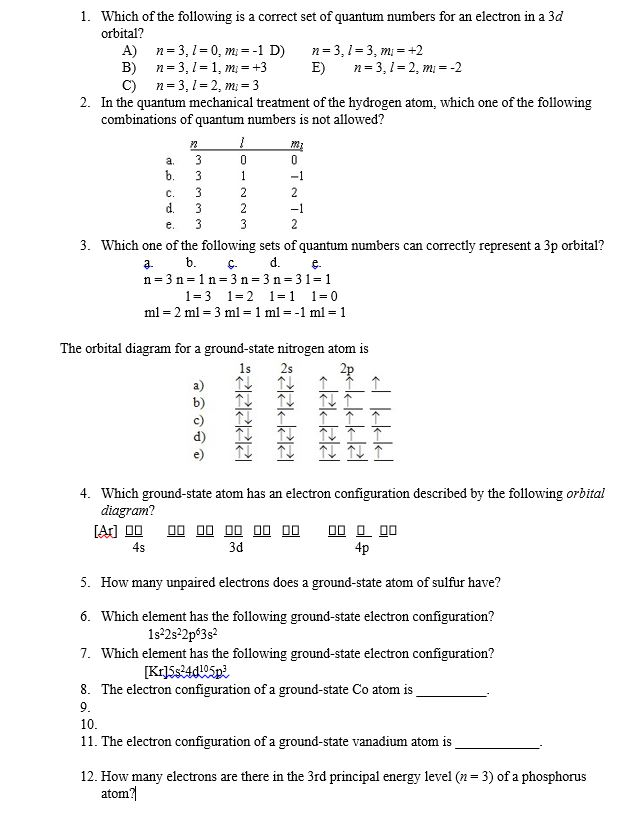 Solved 1 Which Of The Following Is A Correct Set Of Quan
Solved 1 Which Of The Following Is A Correct Set Of Quan
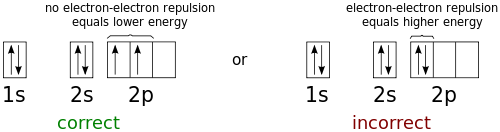 High School Chemistry Orbital Configurations Wikibooks
High School Chemistry Orbital Configurations Wikibooks
What Is The Bond Order For N2 Quora
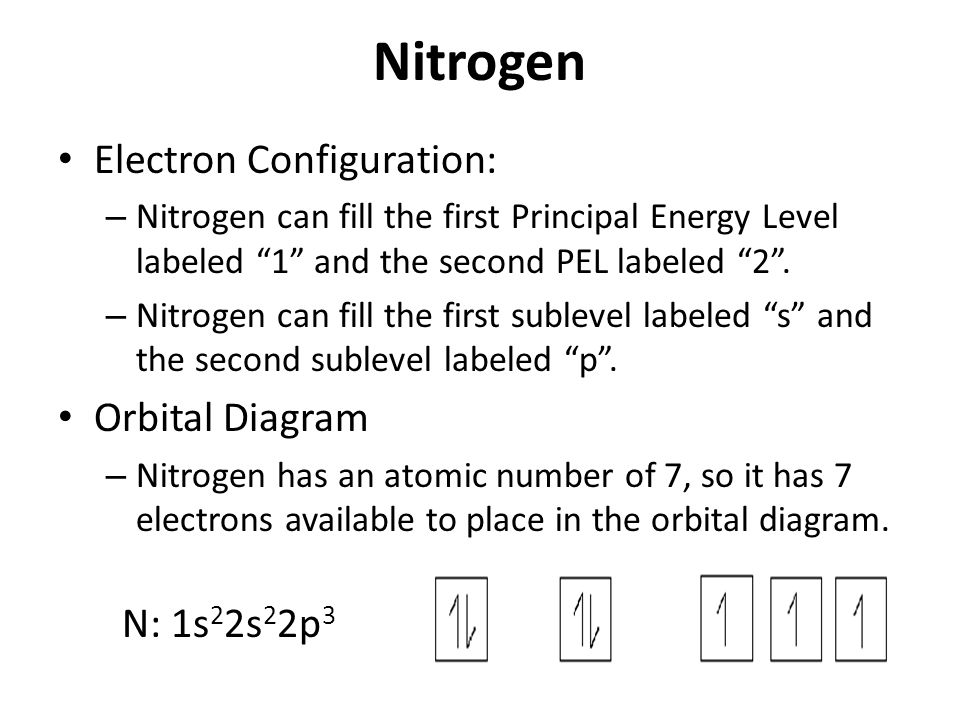 Electron Configuration And Orbital Diagrams Ppt Video
Electron Configuration And Orbital Diagrams Ppt Video
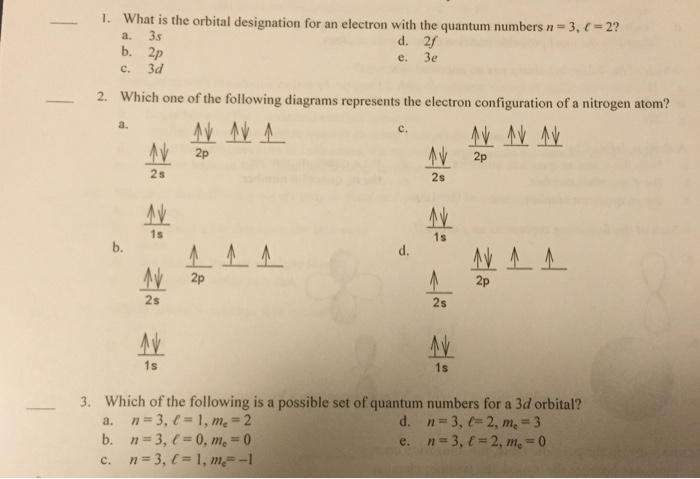
 Electron Configuration Worksheet
Electron Configuration Worksheet
Sparknotes Atomic Structure Electron Configuration And



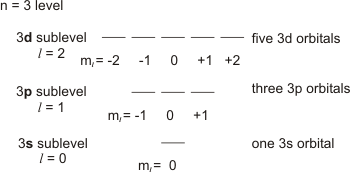

Belum ada Komentar untuk "Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The 3rd Electron Of The N Atom"
Posting Komentar