Molecular Orbital Diagram Khan Academy
The simplest atomic orbital is the spherical 1s orbital of hydrogen. Any advice on if its being worked on or anything would be awesome as well.
It has a hydrogen here 1s orbital hydrogen here 1s orbital.

Molecular orbital diagram khan academy. Using a wealth of examples to depict molecular orbitals mos formed by the linear combination of atomic orbitals lcao she concludes with heteronuclear diatomics. The d orbital makes a double dumbbell or crisscross shape in the x y or z plane. That is by adding and subtracting them.
Just so you get a little bit more notation. Professor sylvia ceyer covers the molecular orbital theory beginning with a discussion of some key topics including bonding orbitals antibonding orbitals electron configurations and bond order. Molecular orbital theory holds as its name suggests that electrons reside in molecular orbitals that are distributed over the entire molecule.
Atomic valence electrons shown in boxes on the left and right fill the lower energy molecular orbitals before the higher ones just as is the case for atomic orbitals. Molecular orbital theory khan academy help center molecular orbital theory follow sanjay d souza and i think that a step by step overview by khan academy would really make a difference 1 molecular orbitals of ethene. You were introduced to the shapes the orbitals make in another video.
The p orbital makes a dumbbell shape in either the x y or z plane. So this is how the hydrogen orbital and the carbon orbitals get mixed. A molecular orbital extends over more than one atom.
I second this request although its a year later. But just to review the s orbital makes a circle or sphere. We then have a molecular orbital.
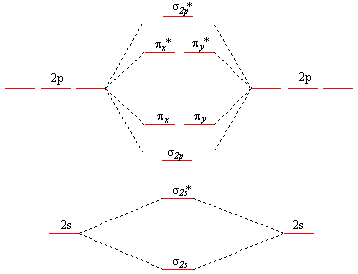
At yukiko sorry for replying a year later as well but just wanted to clarify that there are only lectures on atomic orbitals as well as hybrid. An orbital is a region in space where an electron is most likely to be found. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h 2.
Quantum mechanics specifies that we can get molecular orbitals through a linear combination of atomic orbitals. You wont necessarily find it there 100 of the time. The hydrogens 1s orbital bonds with well each of the hydrogens 1s orbital bonds with each of the carbons sp3 orbitals.
Jpg when two h atoms get close enough their orbitals merge to include both nuclei.
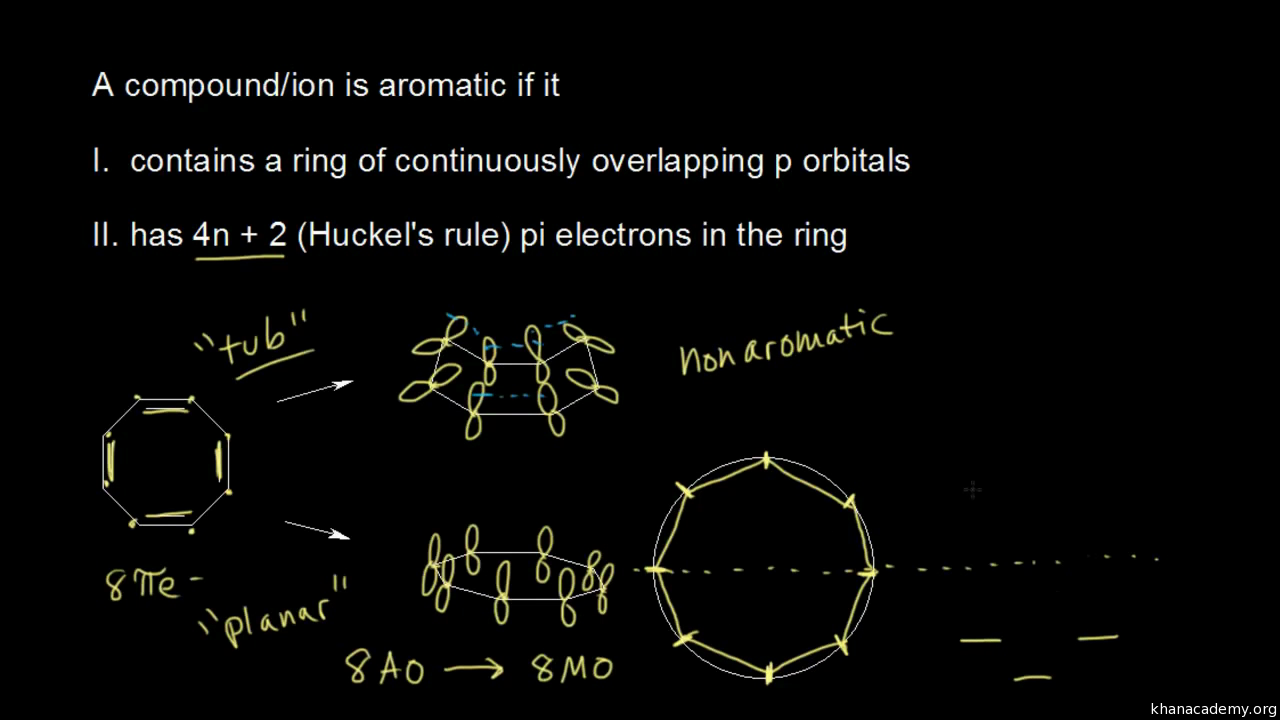
 The Pi Mos Of Benzene And Hexatriene
The Pi Mos Of Benzene And Hexatriene
F Orbital Diagram Example Write Full For F Ion Shape
 The Diels Alder Reaction And Frontier Molecular Orbitals
The Diels Alder Reaction And Frontier Molecular Orbitals
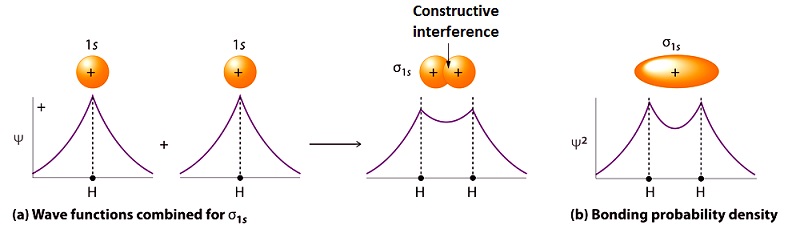 Learn Sigma Pi And Delta Bonding Molecular Orbitals Meaning
Learn Sigma Pi And Delta Bonding Molecular Orbitals Meaning
 Linear Combination Of Atomic Orbitals Wikipedia
Linear Combination Of Atomic Orbitals Wikipedia

 Molecular Orbital Wikivisually
Molecular Orbital Wikivisually
 Orbital Diagram Of Carbon Tattoos Teaching Chemistry
Orbital Diagram Of Carbon Tattoos Teaching Chemistry
What Is The Difference Between Bonding And Antibonding
 Chemical Bonding Molecular Orbitals Of H2 And He2
Chemical Bonding Molecular Orbitals Of H2 And He2
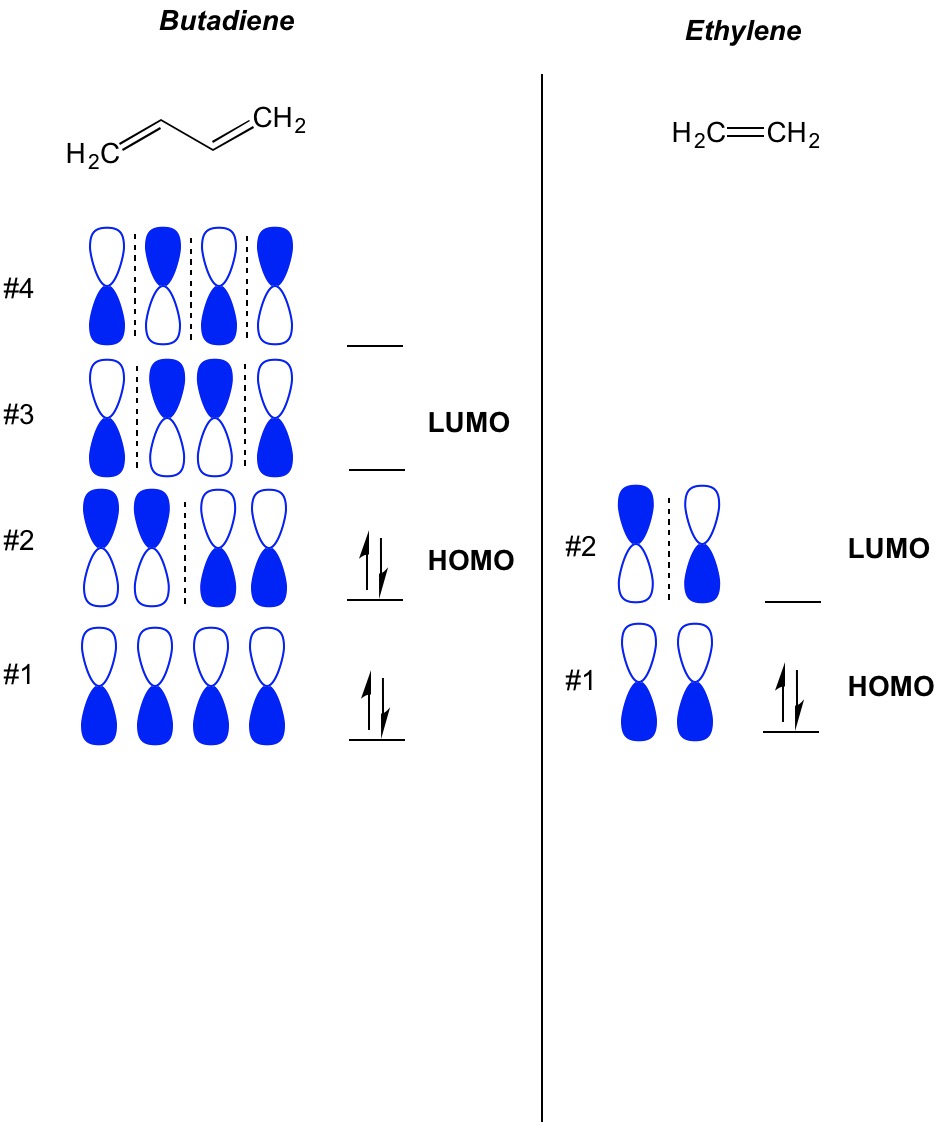 Mo Theory Cycloaddition Organic Chemistry Homo Lumo
Mo Theory Cycloaddition Organic Chemistry Homo Lumo
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
 Molecular Orbitals And Hybridizations Organic Chemistry
Molecular Orbitals And Hybridizations Organic Chemistry
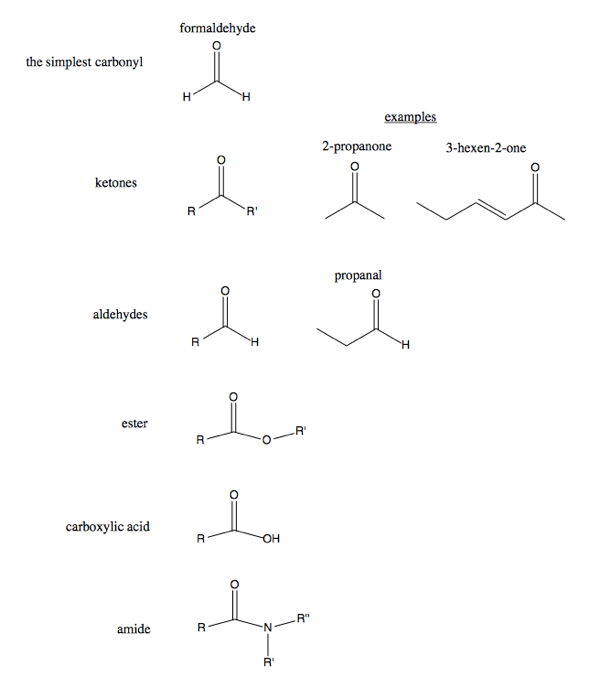 Organic Chemistry 03 Bonding Atomic Orbitals And
Organic Chemistry 03 Bonding Atomic Orbitals And
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
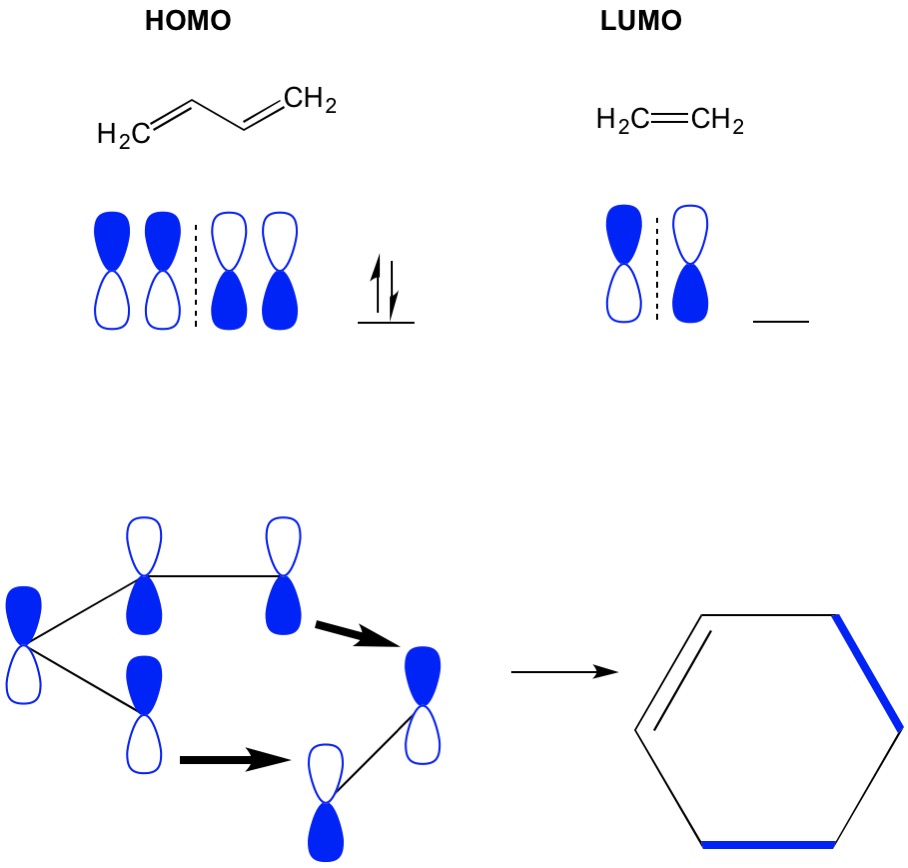 Mo Theory Cycloaddition Organic Chemistry Homo Lumo
Mo Theory Cycloaddition Organic Chemistry Homo Lumo
 12 Awesome Orbital Diagrams And Electron Configuration
12 Awesome Orbital Diagrams And Electron Configuration
 Lewis Diagrams S2 Wiring Diagram Schematics
Lewis Diagrams S2 Wiring Diagram Schematics
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
The Best Free Molecular Drawing Images Download From 66

 Construct The Orbital Diagram For F Sapling Wiring Schematics
Construct The Orbital Diagram For F Sapling Wiring Schematics
 Orbital Diagram Ti List Of Wiring Diagrams
Orbital Diagram Ti List Of Wiring Diagrams
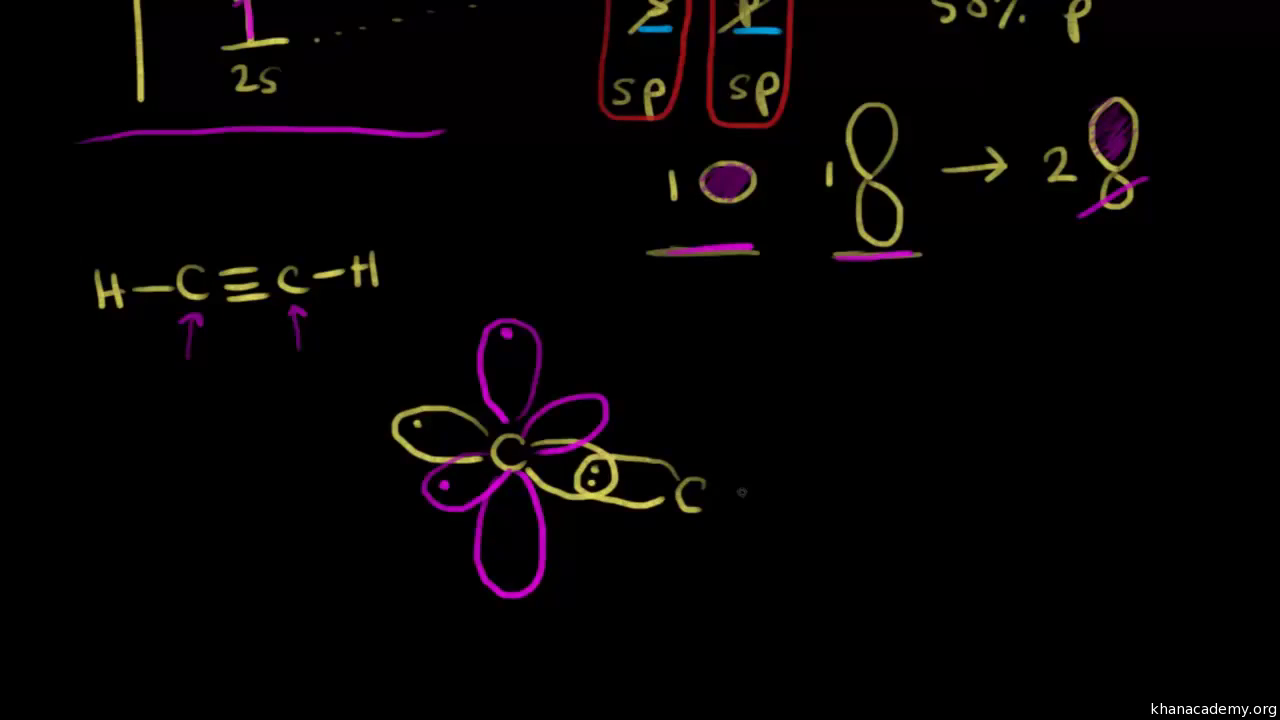
Belum ada Komentar untuk "Molecular Orbital Diagram Khan Academy"
Posting Komentar