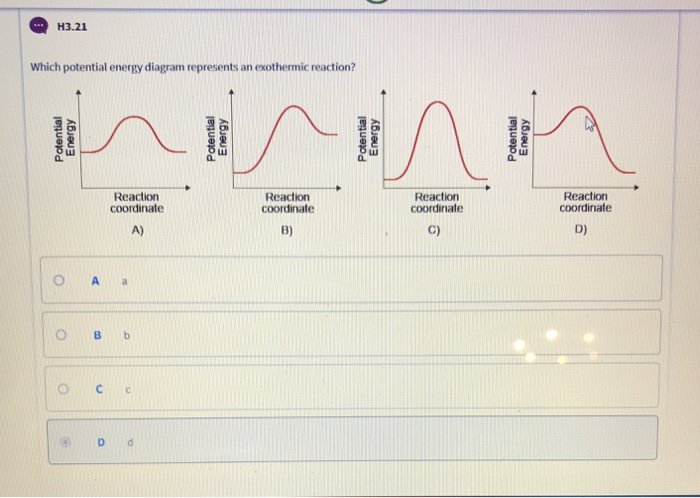
What Is The Activation Energy For The Reaction In This Energy Diagram
This state is also known as an activated complex. For this reason the activation energy of a reaction is sometimes referred to as the activation energy barrier.
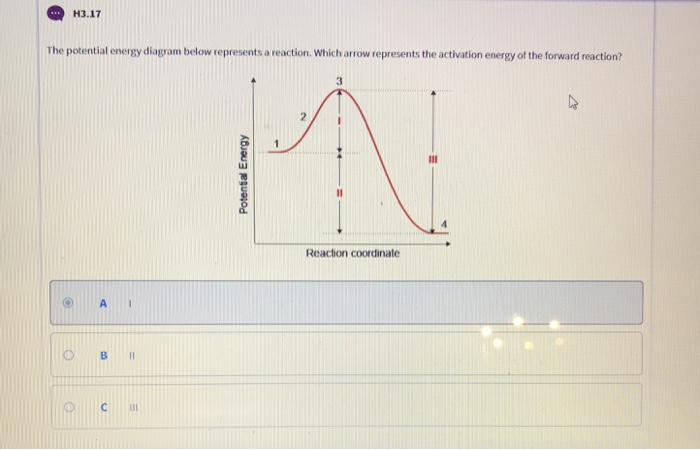
 Solved H3 17 The Potential Energy Diagram Below Represent
Solved H3 17 The Potential Energy Diagram Below Represent
The energy of the reactants of the reverse reaction is 13 kj the activated complex is still at 83 kj.
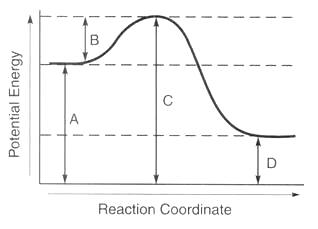
What is the activation energy for the reaction in this energy diagram. The height of this energy barrier you may recall is called the activation energy δ g. Select all that apply a catalyst lowers the activation energy. Activation energy is the difference between the starting amount of energy of the reactants and the energy of the activated complex.
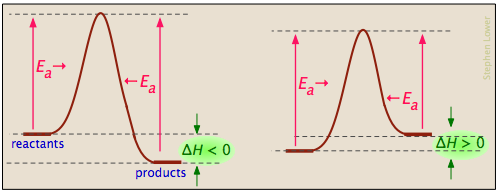
It also shows the effect of a catalyst on the forward and reverse activation energy. If the reaction were to proceed in the reverse direction endergonic the transition state would remain the same but the activation energy would be larger. First an energy barrier must be overcome to get to the product side.
The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. The higher the energy hill the slower the reaction.
It describes the relationship of the enthalpy of a reaction with the potential energy difference of the. The activation energy diagram is drawn as a hill because there is a large amount of energy needed to form the unstable transition state viewed as the upward slope on the diagram. The activation energy shown in the diagram below is for the forward reaction reactants products which is exergonic.
At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. The activation energy is what determines the kinetics of a reaction.
 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
 Reaction Coordinate Diagrams College Chemistry
Reaction Coordinate Diagrams College Chemistry
 Solved H3 17 The Potential Energy Diagram Below Represent
Solved H3 17 The Potential Energy Diagram Below Represent
The Arrhenius Law Activation Energies Chemistry Libretexts

 How Can I Draw A Simple Energy Profile For An Exothermic
How Can I Draw A Simple Energy Profile For An Exothermic
 Pin By Noah Melon On Games Gcse Chemistry What Is Energy
Pin By Noah Melon On Games Gcse Chemistry What Is Energy
 Activation Energy Higher Chemistry Unit 1
Activation Energy Higher Chemistry Unit 1
 Which Reaction Coordinate Diagram Represents A Reaction In Which The Activation Energy Ea Is 50 Kj Mol 1 And The Dhrxn Is 15 Kj Mol 1
Which Reaction Coordinate Diagram Represents A Reaction In Which The Activation Energy Ea Is 50 Kj Mol 1 And The Dhrxn Is 15 Kj Mol 1
Powerschool Learning 8th Grade Science Activation Energy
Er10 Temperature Rate And Potential Energy Diagrams
 What Is Activation Energy For The Forward Reaction
What Is Activation Energy For The Forward Reaction
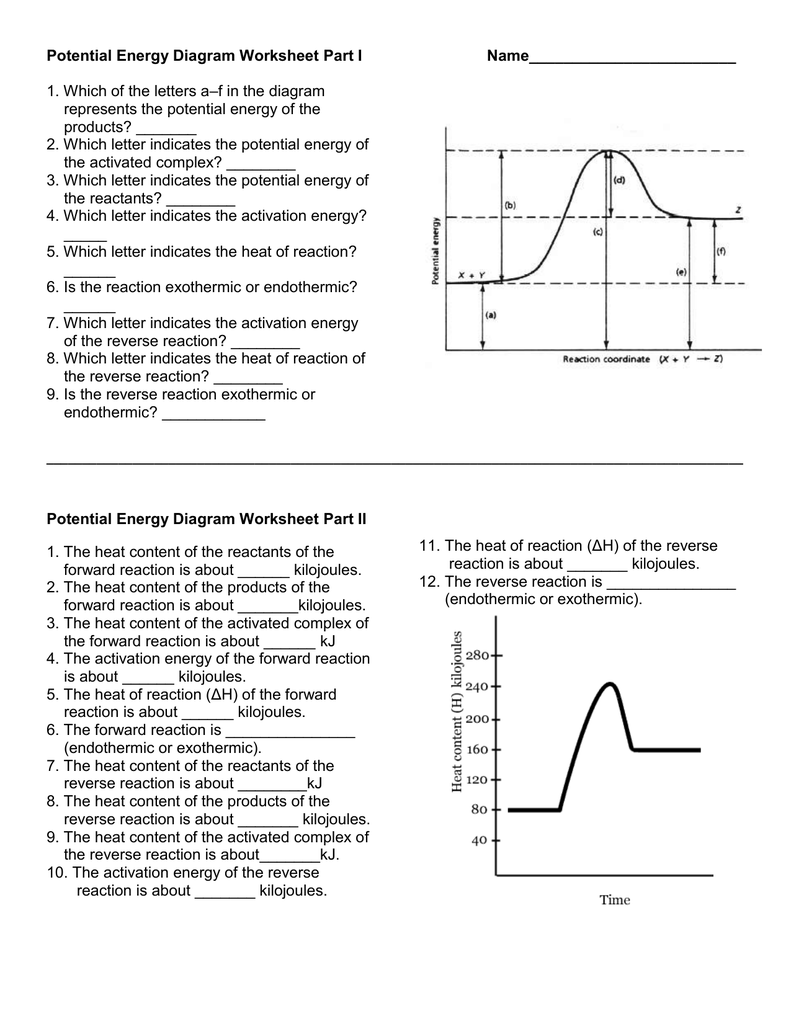 Potential Energy Diagram Worksheet Part I
Potential Energy Diagram Worksheet Part I
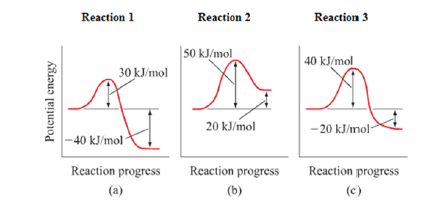 Solution The Potential Energy Profiles Fo Clutch Prep
Solution The Potential Energy Profiles Fo Clutch Prep
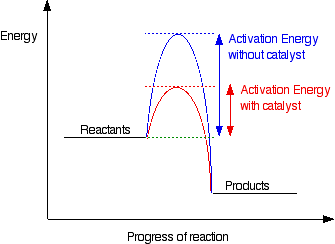 A Look At Energy Profiles For Reactions Chemistry Libretexts
A Look At Energy Profiles For Reactions Chemistry Libretexts
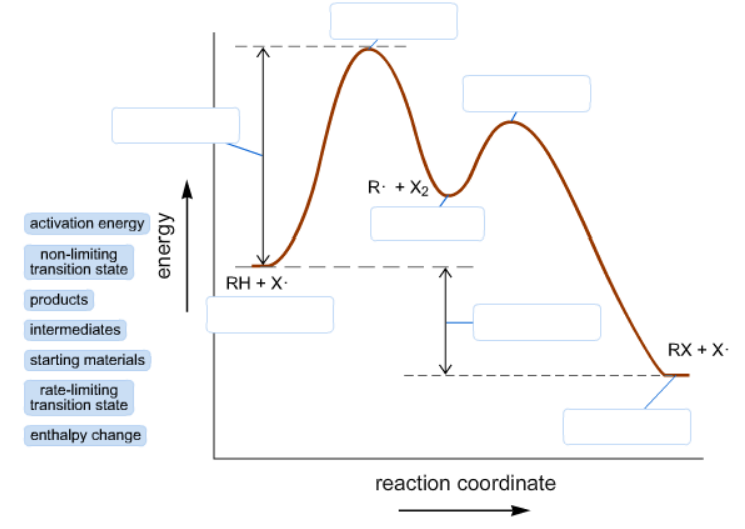 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep
 How Can I Find The Activation Energy In Potential Energy
How Can I Find The Activation Energy In Potential Energy
 Endothermic Reaction Diagram Energy Diagram For A
Endothermic Reaction Diagram Energy Diagram For A
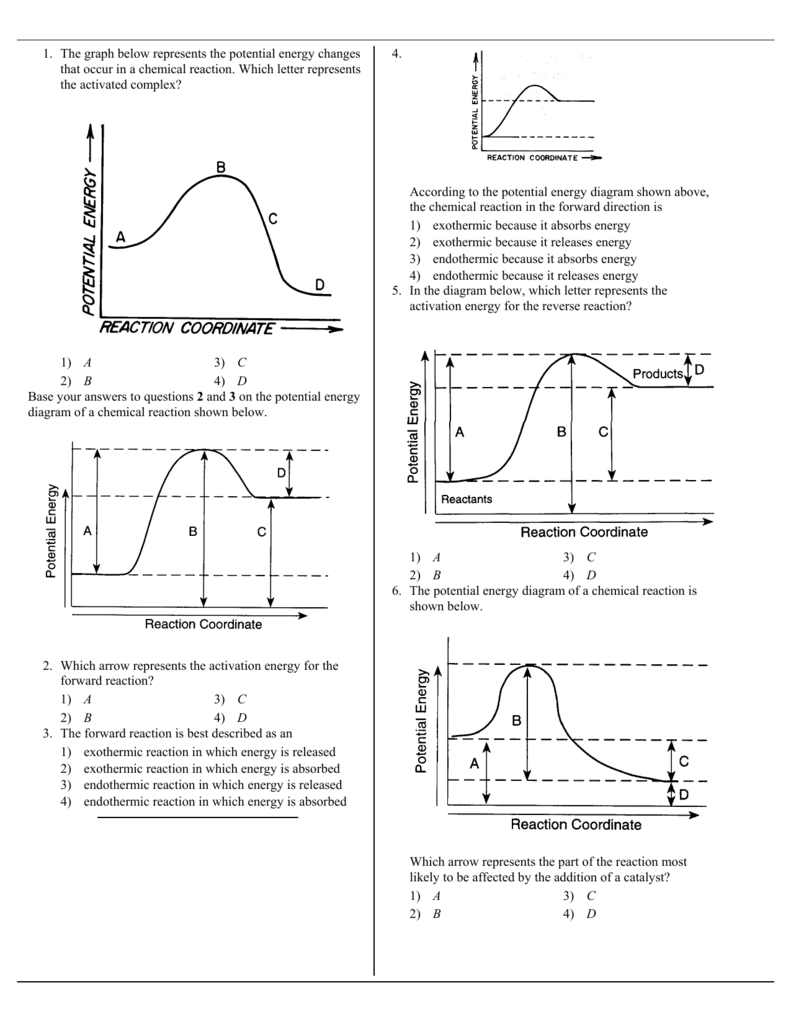 1 The Graph Below Represents The Potential Energy
1 The Graph Below Represents The Potential Energy
 Energy Diagram For A Simple Reaction The Same Reaction With
Energy Diagram For A Simple Reaction The Same Reaction With
Mcat General Chemistry Question 62 Answer And


Belum ada Komentar untuk "What Is The Activation Energy For The Reaction In This Energy Diagram"
Posting Komentar